
Evidence based treatment options for common knee injuries in runners
Introduction
Injury is a key barrier in many runners, accounting for a 19.4% to 79.3% lower extremity injury rate (1). With over 40 million people in the United States running regularly, this percentage can represent a significant amount of runners with injuries (2). Nearly half of those 40 million runners experience some type of pain or injury annually. The knee is the most common area of injury in runners, the knee joint is affected by three main injury types including patellofemoral pain syndrome (PFPS), iliotibial band friction syndrome (ITBFS), and patellar tendinopathy (PT).
PFPS is the most common musculoskeletal overuse injury in active adults. With a prevalence reported up to 19–30% of female runners, and 13–25% of male runners (1,2).
PFPS is the most prevalent running-related injury. PFPS is characterized by a gradual onset of anterior or retro patellar knee pain typically experienced under loading and compressive forces. Activities involving significant quadriceps demand with knee flexion such as running, squatting, hopping, stair climbing, and even prolonged sitting can onset symptoms (3).
ITBFS is another one of the commonly diagnosed injuries in runners. This syndrome is characterized by pain during loading of the lower extremity over the lateral aspect of the knee, with pain over the lateral knee when the knee moves from flexion into extension.
PT is a condition that primarily affects 15 to 30 years old athletes, with males affected more than females. This condition is seen in runners, but is also notably seen in events with repetitive jumping and loading to the quadriceps during sports such as volleyball, basketball, tennis, and football. PT is most notably characterized by pain at the attachment of the patellar tendon at the inferior pole of the patella. PT is activity dependent and usually only symptomatic in high energy activities such as running and jumping.
These three conditions represent a large section of knee pain in runners. Review of the evidence and knowledge towards treating these conditions can improve clinical effectiveness and efficiency in treatment.
PFPS
Patellofemoral pain is thought to be caused by abnormal tracking of the patella in the trochlear groove. Several factors including lower extremity malalignment, muscular imbalance or insufficiency, decreased flexibility, patellar hypermobility, faulty running mechanics, and over activity can attribute to patellofemoral pain (4,5).
Alignment factors associated with PFPS include femoral neck anteversion, genu valgum, larger Q angle, knee hyperextension, tibial varum, and excessive rearfoot pronation (6,7). Patellar alignment, shape, and joint congruency have also been noted to be predisposing factors for PFPS. Further investigation is needed into lower extremity alignment factors, however, as current scientific data is conflicting and no norms for the above values have been established.
Muscular imbalances and weaknesses are also common findings in those with PFPS. Two primary theories of muscular imbalance exist, one focusing primarily at the knee and the other at the hip. The knee concept postulates that quadriceps weakness, specifically at the vastus medialis obliquus (VMO) is the primary cause of patellar maltracking. The VMO is overpowered by lateral structures including the iliotibial band (ITB), lateral retinaculum, and vastus lateralis (VL). This imbalance leads to lateral patellar tracking and tilting, causing compression and pain (8). The hip theory suggests that decreased eccentric hip abductor and external rotator strength causes a relative femoral internal rotation and/or hip adduction moment. This causes increased compressive forces of the patella in the trochlear groove with dynamic movements such as running or squatting (9).
Research has shown decreased quadriceps, hip abductor and hip rotator strength may be related to the development of PFPS (10-15). Muscular weakness can be concentric, but poor eccentric strength, predominately at the hip abductors and adductors has also been shown in those with patellofemoral pain (15). Other studies have refuted weakness of these muscles as a cause of PFPS primarily as it is difficult to determine if weakness causes the patellofemoral pain, or if it is actually a result of that pain. Regardless, pain and weakness appear to coincide with PFPS and the muscles most likely responsible are the quadriceps, hip rotators, and hip abductors.
Decreased muscular or myofascial flexibility is also an important contributing factor causing patellofemoral compression. Primary structures involved include the hip flexors and quadriceps (16-18). Decreased hip flexor and quadricep flexibility causes compression via the posterior pulling of the patella into the trochlea (19). Tightness in the tensor fasciae latae and its dense fascial insertion, the ITB, has also been shown in those with PFPS as these structures will pull the patella laterally causing compression. Finally, the hamstrings can also play a role in the development of patellofemoral pain as they will pull the tibia posteriorly on the femur increasing the patellar compression and stress especially with flexion exercises such as squatting or stair descending (20).
Specific faulty mechanics with running have also been shown to predispose a runner to developing PFPS. In a study by Noehren et al., female runners with PFPS demonstrated increased hip adduction and internal rotation angles during midstance (Figure 1) compared to healthy runners (21). In male runners, Willy et al. demonstrated those with PFPS had significantly more contralateral pelvic drop (Figure 2) and significantly less hip adduction than females with PFPS (22). Increased ground reaction forces can also be responsible for developing PFPS. A pilot study by Davis et al. showed that runners with a history of patellofemoral pain demonstrate a higher impact peak and loading rate, indicating that increased mechanical forces with running can lead to injury (23). Each of these factors on their own or in combination can lead the patellofemoral joint to increased shearing forces and pain.

Errors in training account for more than 60% of running-related injuries. Those presenting with PFPS often report increases in running mileage and/or speed or changes in running environment including increased stair or hill training (5). These training factors in combination with decreased recovery time, sleep and nutrition deficits, and increased overall stress further predispose the runner to developing injury.
Treatment
Numerous interventions exist for managing patellofemoral pain. This can make effective treatment difficult to navigate. Our recommendations coincide with those of the International Patellofemoral Research Retreat. These recommendations were developed as the evidence-based 2018 consensus statement focusing on exercise therapy and physical interventions (e.g., orthoses, taping and manual therapy) for patellofemoral pain. Recommendations by the expert panel on patellofemoral pain include:
- Exercise therapy to decrease pain and improve function. Focus on both hip and knee centered exercises rather than the knee alone;
- Combined interventions, including taping, orthotic management, and/or manual therapy should be used in conjunction with prescriptive exercise to decrease pain;
- Foot orthoses are recommended to decrease pain in the short term;
- Patellofemoral, knee, and lumbar mobilizations are not recommended in isolation;
- Electrophysical agents are not recommended including laser, ultrasound, or phonophoresis therapies (24).
One must first determine the primary driving factors for the patient’s symptoms to most effectively treat the patient. Once a thorough examination has been performed, a specific, individualized, and focused treatment approach should be enacted.
Manual therapy
Manual therapy techniques utilized to treat patellofemoral pain include joint mobilizations, soft tissue mobilizations (with and without instrument assist), manual stretching, and trigger point dry needling (TPDN). A systematic review performed by Jayaseelan et al. studying the effect of joint mobilizations found that joint mobilizations were effective in decreasing pain when used in conjunction with other interventions. It also determined the most effective mobilizations were directed at the knee complex rather than the lumbopelvic region or other surrounding joints (25).
Numerous types of soft tissue techniques can be utilized in the treatment of PFPS. These techniques include instrumented assisted soft tissue mobilization (IASTM) such as Graston or augmented soft tissue mobilization (ASTYM) which are used to treat dense connective tissue adhesions. Manual soft tissue mobilizations including massage, myofascial release, and ischemic compression of trigger points are also utilized. The evidence supporting these techniques in isolation is limited, however, incorporating soft tissue therapies can decrease muscular tone, improve blood circulation, and introduce a neurophysiological effect to decrease pain.
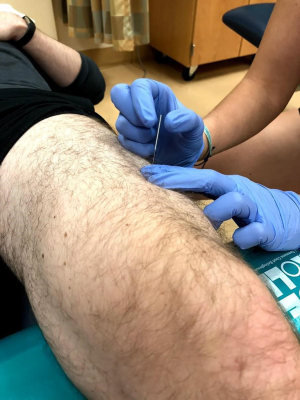
TPDN (Figure 3) is typically used in conjunction with exercise therapy and other modalities to decrease pain. Active trigger points in the quadriceps, specifically the vastus medialis and rectus femoris can refer pain to the anterior knee and mimic or exacerbate existing patellofemoral pain symptoms (26). It is theorized that treating these trigger points can help decrease referred pain. TPDN can also be used in conjunction with electrical stimulation to increase specific muscle activation or fatigue overactive muscles. TPDN should be followed by specific exercise or neuromuscular re-education targeted at the musculature treated or movement patterns to be trained in order to optimize benefits. While in the clinic setting, TPDN appears to be effective in some patients for muscle activation and pain control, the evidence is lacking. One study by Sutlive et al. determined no significant change when incorporating dry needling vs. sham needling in the management of PFPS (27). Further research is needed on the application of dry needling in treating patients with patellofemoral pain.
Modalities
Taping
Patellar taping (Figure 4) is commonly used in conjunction with manual and exercise therapies in the management of PFPS. Taping is predominately used to help decrease pain (28). Other studies show it can also help with patellar alignment and muscle activation (29,30). As patellar hypermobility has been shown as a predisposing factor for developing PFPS, taping can be indicated to promote patellar positioning and decrease pain.
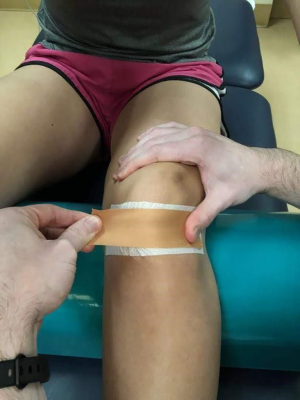
McConnell taping is one common taping technique used. This technique has been proven to both sufficiently pull the patella medially (29) and cause earlier onset of VMO activation (30). This is suspected to help promote medial positioning of the patella to decrease lateral gliding causing shearing and pain from overactive or dense lateral structures.
Overall, the effects of taping, are conflicting, with some studies showing no benefit and others unsure of the mechanisms of improvements noted. The positive changes including decreased pain and improved VMO function are only short-term but can be helpful with acute management of symptoms with functional activity.
Orthotic management
Foot orthoses are another common treatment intervention that have been shown effective for managing patellofemoral pain. Traditionally, orthotics are prescribed to individuals with PFPS due to overpronation of the foot causing suspected hip and knee compensations, leading to patellar shearing and pain (31). Studies are limited, however, linking excessive pronation to PFPS. One study by Vicenzino et al. showed a significantly greater number of individuals reported positive outcomes when issued a foot orthosis, regardless of foot type, in short term improvement (32).
Several studies have looked into developing clinical predictors to determine the effectiveness of orthotics for managing PFPS. One study by Vicenzino et al. developed four predictive variables to include older individuals, shorter individuals, individuals with lower baseline pain scores, and individuals with greater foot mobility as those that benefited most from orthotic management (33). Another study by Barton et al. showed four other predictors for positive outcomes with orthotic management including reduced pain with single leg squat, less usual pain at baseline, decreased ankle dorsiflexion, and poor footwear motion control properties. A combination of 3 out of 4 of these variables significantly increased the probability of improvement of symptoms from 25–78% (34,35).
When considering orthotic management of a patient with patellofemoral pain, one must consider several factors including custom fit versus off the shelf orthotics. Individuals are often issued generic orthotics that may not be appropriate for the individual’s specific foot type. Another consideration is that even custom made orthotics are still often created in a non-weight bearing position, which may cause modifications that can be deemed inappropriate once the individual is walking or running.
It is recommended to consider a patient’s age, height, and baseline pain scores. These factors in concordance with foot and ankle morphometry, pain changes with functional testing such as single leg squatting, and current footwear choice, can help determine if orthotic management will be effective in treating patellofemoral pain.
Therapeutic exercise
Weakness in the quadriceps as well as the hip external rotators and abductors has been related to the development of patellofemoral pain. Historically, rehabilitation programs focused solely on the retraining of the quadriceps musculature with little to no emphasis on the hip. A systematic review of EMG studies showed, however, it is nearly impossible to isolate vastus medialis activation from remaining quadricep muscles (36). One way to improve isolation of the vastus medialis is with electrical stimulation. Combining electrical stimulation directly to the VMO with taping for medial pull can further increase proper activation and decrease muscular imbalance (37). Combining these therapies with quadriceps strengthening exercises promote strength gains.
In addition to knee strengthening, hip strengthening is integral for a comprehensive rehabilitation program. One study by Ferber et al. looked at strengthening regimens focused on the knee versus the hip. Both showed decreased pain and improved strength of addressed musculature but the hip group had an earlier resolution of pain with greater strength gains (38). Increasing hip rotation and abduction strength both concentrically and eccentrically can improve femoral positioning on the tibia with dynamic activities and decrease patellofemoral stress. This especially important with weight bearing exercises to improve loading mechanics with functional movements.
Muscular flexibility should be assessed with special attention to the hip flexors and quadriceps as well as the tensor fascia latae (TFL) and hamstrings. Upon determining which structures are involved, have the patient perform the proper stretch (Figures 5-7) for 4–6 min per group. This can be broken down into 60–90 s intervals but should be no shorter than 60 s (39).
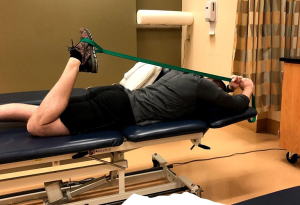
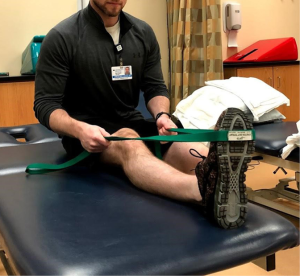
Foam rolling or self-soft tissue mobilization can also be effective in decreasing neuromuscular tone in the involved musculature. Several studies have shown foam rolling is effective in improving short term flexibility of the quadriceps, hamstrings, and hip flexors in healthy subjects (40-42), however, it is unknown as to how long these effects last. Further investigation is needed to determine long term effects of foam rolling; however, it can be a useful tool in decreasing muscular tone and pain prior to or following exercise.
Neuromuscular re-education
In conjunction with a strengthening program, coordination and motor control deficits should also be addressed. Focus should be on single leg hip and knee control, multi-chained movements, and plyometric loading and propulsion. The use of external cues including auditory and visual cues have been shown to be very effective with running rehabilitation (Figure 8) (43,44). These external cues are essential for motor learning and should be consistently utilized with both plyometric and running retraining. A study by Willy et al. demonstrated that mirror retraining effectively trained proper running mechanics with carry over up to 3 months post re-training. These learned strategies were also translated into other activities including single leg squatting and step downs, activities that often also cause patellofemoral pain (43).
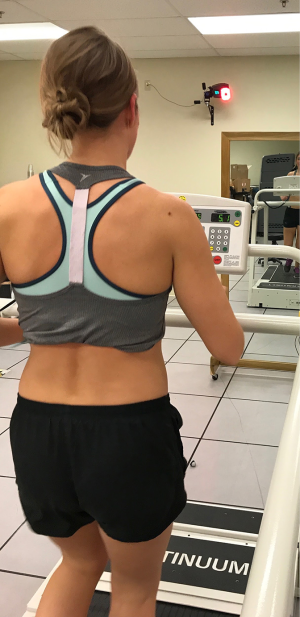
Bottom line
Running analysis
Runners are likely to demonstrate increased hip internal rotation and adduction along with increased contralateral pelvic drop in midstance. Runners are also likely to demonstrate higher increased ground reaction forces with loading.
Manual therapy
Manual therapy should be used in conjunction with a strengthening and neuromuscular re-education program. Manual therapy should focus on improving soft tissue restriction particularly along lateral structures and promoting joint mobility particularly at the knee itself if hypomobility or range of motion deficits are evident.
Modalities
Taping and orthotic management may be beneficial in the short term to decrease pain with functional activities, however, should not be depended upon for long-term treatment of symptoms.
Therapeutic exercise
A strengthening program should focus on both quadriceps and a hip rotation/abduction strengthening and include concentric and eccentric components. It should also include an extensibility and/or mobility component specific to the areas lacking flexibility.
Phase 1: low load muscular activation exercises
- Side plank;
- Quadruped fire hydrants (Figure 9);
- Lateral step down;
- Three-way straight leg raise.
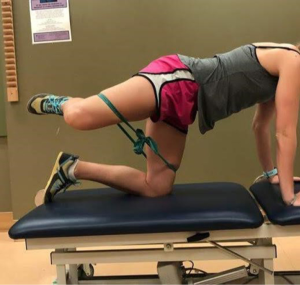
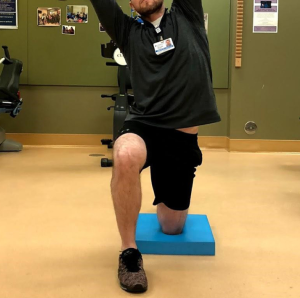
Phase 2: multi-chained control and coordination exercises
- Single leg squats;
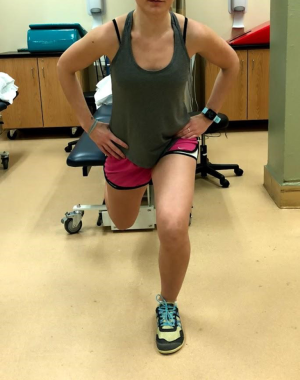
- Split squat (Figure 10);
- Step up plus (with or without overhead press);
- Three-way slider lunges.
Phase 3: plyometric loading and propulsion exercises
- Double leg squat jumps;
- Double leg box jumps up/down;
- Single leg hop downs;
- Single leg forward hops.
Neuromuscular re-education
A motor retraining program should coincide with the strengthening component emphasizing single leg control with dynamic and plyometric movements. External cues should be utilized when possible with both plyometric and running retraining to aid motor learning of improved hip and knee control. Simple verbal cues to “land softer” or “closer to body” may decrease ground reaction forces and improve foot strike position to decrease load forces.
Conclusions
Patellofemoral pain is highly common in active adults, specifically runners. Determining the root cause of the patellofemoral irritation is key to effectively treating it. One must first perform a thorough subjective exam of the patient with emphasis on notable changes in running frequency, intensity, and total mileage. It is also important to note changes or trends in sleeping patterns, nutrition and hydration habits, and potential external stressors that could be exacerbating symptoms. Next, one must perform a comprehensive musculoskeletal exam looking at strength and flexibility of isolated muscle groups, postural alignment, joint mobility, and foot structure. Finally, one must also evaluate the patient’s potential biomechanical deficits with functional activities including squatting, single leg squatting, step downs, and running.
Once primary causes of the patellofemoral pain are identified, it is important to treat those deficits with utilizing a comprehensive approach. A strengthening and motor retraining program should be prioritized with adjunct manual therapies and modalities added as needed based on the individual needs of the patient.
ITBFS
ITBFS is another one of the most common overuse injuries in runners. A systematic review in the American Journal of Sports Medicine estimated an incidence of 5% to 12% in runners, and is the most common lateral knee running related injury (45).
Different opinions exist on the etiology of ITBFS. One theory states abnormal compression of the distal band as it inserts into periosteum over the lateral epicondyle causes the lateral knee pain. This area is abundant in fat, blood vessels, nerves, and Pacinian corpuscles. Continuous compression would irritate and inflame this area. The second theory states the friction of the posterior ITB becomes inflamed as it travels over the lateral epicondyle of the femur in an anterior to posterior direction with knee flexion (46). Histological studies of knee joints with ITBFS show inflammation, soft tissue thickening of the IT band or fibrosis, and excess lateral fluid of the knee joint (47). This is consistent with chronic tendinopathic changes that lead to thickening and deformation of the distal ITB.
Common diagnostic factors of iliotibial band syndrome (ITBS) are pain and swelling over the lateral knee joint. Pain is reproduced as the knee extends from 90 to roughly 30 degrees as the ITB is compressed at the lateral femoral epicondyle. This is also known as the Noble Compression Test. Past studies have shown weakness in abductors (gluteus medius and upper fibers of gluteus maximus) in runners with ITBS (48). Increased mileage was the most common attribute associated with ITBS according to Noble (49).
From a running kinematics perspective, ITB strain occurs during the initial loading phase and deceleration stance phases. Biomechanical analysis has shown increased hip adduction, knee internal rotation, and femur external rotation in females with ITBFS compared to age-related controls (50). In males, increased knee adduction and hip internal rotation were found compared to controls. Further studies have shown no elongation or strain of the ITB, but strain rate or quicker movement into elongation were significantly higher in runners with ITBFS vs. the uninvolved limb (51). Ipsilateral trunk flexion to the involved side during stance has shown to be a prevalent compensation and possible method to reduce strain on the ITB. One way researchers measures the tension or hardness was through a method called shear wave elastography. Using this method, researchers looked at multiple movements of the pelvis, and lower extremity. Measurements that showed the most ITB hardness happened with pelvic drop and trunk lean away, and decreased with pelvic rise, and trunk lean towards affected side. Narrow than preferred foot placement has also been associated with possible increased ITB strain. This can often be seen with a cross-over gait pattern (52).
Treatment
Manual therapy
Research involving manual therapy techniques for the treatment of IT band syndrome is lacking. Due to the many points of contact between the ITB to the biceps femoris, VL, TFL, and gluteus medius and maximus, myofascial mobilizations to the musculature attaching to the IT band could be beneficial. Due to the proximal structures the ITB tensions, lumbar/sacral and pelvic dysfunctions should be also addressed. With evidence that chronic IT band syndrome results in deformation and thickening of the distal tendon, manual therapy techniques involving reformation of a degenerated tendon, such as instrument IASTM may be of use (53). A randomized controlled trial showed cross-friction massage had no additional effect in daily pain and running pain levels versus typical physiotherapy (54). Stretches involving the ITB and muscular complex have shown to decrease the adduction moment in elite athletes during running and increase mobility of the ITB complex, particularly when adding an overhead UE abduction with the stretch (55).
Modalities
Studies do show a role for anti inflammatories and their role for management of pain in ITBS. A 1991 double blinded study showed decreased pain with ITBS short term with the application of NSAID’s (56). During acute onset ITB syndrome, corticosteroid injection with application of ice and rest, showed significant decrease in pain vs. control in a 14-day period (57).
Therapeutic exercise
Several studies have found decreased hip abduction strength to be associated with ITBS in runners. Additionally, studies focusing on strengthening of hip abduction musculature as part of a multimodal program, show an improvement in pain and function. Training the hip external rotators and the gluteus maximus is important due to weakness and the propensity for loss of control of closed chain hip internal rotation in males with ITBS. Emphasis on eccentric control is vital on these muscle groups as ITB strain is shown to occur during the deceleration phase of stance. However, Willy et al. performed movement retraining of a single-leg squat task plus a hip strengthening program to runners. Only the single leg squat task improved in form with no significant changes in running form. This leads to the hypothesis that actual movement retraining of the running form is crucial to pair with hip strengthening (58).
Neuromuscular re-education
Neuromuscular retraining is appropriate even in the acute phase. Studies have shown walking with slight trunk flexion, or walking with knees together to decrease knee adduction moment, may be beneficial especially for male runners. Introducing the concept of hip control during ambulation in the acute phase may be beneficial for increased carry-over into running biomechanical training. Two-legged landing training involving a soft landing and increased hip strategy, may also be beneficial.
Manipulating cadence may also be indicated. A study by Heiderscheit et al. showed an increased cadence by 5% to 10% on healthy runners can decrease hip adduction angle, peak adduction, and hip internal rotation moments. This can potentially decrease the tension placed through the ITB (59). A small study showed this increase in cadence does not decrease running efficiency in healthy runners and may be an effective training tool for runners with ITBS (60). Case studies have shown success with cadence training with runners with ITB syndrome, but further controlled studies are lacking.
Bottom line
Running analysis
Runners with clinical signs and symptoms consistent with this diagnosis are more likely to run with the following strategy: increased pelvic drop away from the involved side, ipsilateral trunk flexion towards the involved side, and a narrower stance. For males: increased knee adduction and hip internal rotation. For females: increased hip adduction, knee internal rotation, and femur external rotation.
Manual therapy
Manual techniques should address lumbopelvic dysfunction if indicated after assessing lumbopelvic stability. One should address muscular extensibility of the lateral iliotibial chain (TFL, VL, gluteus medius/maximus, biceps femoris) using trigger point release, dry needling, soft-tissue mobilization (instrument assisted as indicated), and stretching. Points of inflammation and fibrosis of the ITB as it crosses lateral epicondyle and attaches to the lateral retinaculum must also be addressed with manual techniques and modalities to control inflammation as needed.
Therapeutic exercise
Exercises should be broken into three phases including open kinetic chain (OKC), closed kinetic chain (CC), and high-impact exercises. Since ITBFS is shown to occur during a loading, decelerating phase, the most advantageous position for strengthening would be in a closed-chain loaded position. Once closed-chained hip strength improves, training should progress towards activation of these muscles in a high impact activity such as hopping, jumping, and landing.
Phase 1: strengthening (open chain)
Exercises should focus on proper activation of all musculature supporting the lateral chain during the stance phase of walking or running:
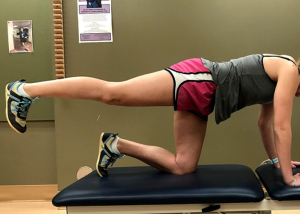
- Clamshells;
- Mule kicks (with knee extended and flexed) (Figures 11,12);
- Single-leg bridge;
- Side-step with band proximal to knees.

Phase 2: closed chain
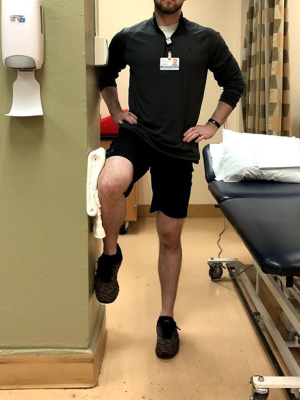
Progression to phase 2 involves minimal pain (less or equal to 3/10) with the following exercises.
- Single-leg stance with uninvolved lower extremity (LE) abduction against wall (Figure 13);
- Hip hikes on involved side;
- Single-leg squats (Figure 14);
- Single-leg deadlift.

Phase 3: plyometrics
Progress to Phase 3 with reintroduction to running. At this point the patient should be able to perform higher intensity Phase 2 exercises with minimal to no knee pain:
- Bilateral lands off of step in front of mirror emphasizing soft-landing and sitting in a chair to cue increased hip strategy. Progress to single-leg landing;
- Single leg lateral hops in front of mirror emphasizing pelvic and hip control. Progress single-leg hops in multiple directions with agility ladder.
Neuromuscular re-education
Ambulation training should emphasize increased hip control in the acute phase. If a significantly decreased cadence is demonstrated, an increased cadence training program of 5% to 10% can be enacted. Visual cues to decrease hip adduction and internal rotation angles should be given throughout training and gradually removed.
Conclusions
ITB syndrome occurs predominantly in the stance phase of running. When assessing a runner for ITBFS, subjective intake of the runners mileage, shoewear, frequency is crucial, as well as the objective intake of the running technique. It is important to assess muscular strength and extensibility, in addition to trigger point referral patterns of the surrounding musculature attaching to the IT band. Secondary alignment issues of the lumbopelvic chain should be assessed and addressed.
Manual therapy should address points of inflammation, myofascial restriction, active trigger points, and lumbopelvic dysfunctions. Manual therapy should be used in conjunction with a specific exercise prescription progressing from open to closed chain exercises then to higher impact movement retraining with overall goal returning to running.
PT
Patellar tendon pathology passes through a spectrum of phases. Reactive tendinopathy starts as a non-inflammatory response to a tendon that is acutely overloaded. This creates a thickened tendon to resist an increased load (61). As continued overload occurs, tendon dysrepair begins with increased proteoglycan laydown in the tendon and continues to thicken, creating a more disorganized tendon matrix (62). Degenerative tendinopathy with excessive matrix breakdown, and actual cell death in certain areas of the tendon happens as the condition becomes more chronic in nature (63). PT is associated with pain in the inferior pole of the patella and repetitive loading of the quadriceps and the patella-patellar tendon complex. PT tends to be dose dependent, and is exacerbated by overuse, especially with the knee extensors, and is usually not painful in a resting state. Activities that store and release energy from the patellar tendon are the most painful, such as deep squats, jumping, and landing.
Successful treatment is highly dependent on correct diagnosis of the knee condition, as quite often PT may have similar characteristics to PFPS, inflammation of the infrapatellar fat pad, quadriceps tendon tendinopathy, plica pathology, and patellar chondral defects.
Several factors can contribute to the onset of PT including muscular weakness or atrophy, foot posture, decreased motor control, and faulty running mechanics.
Reduced strength and/or atrophy in leg musculature such as the quadriceps, gastroc-soleus, and hip abductors is seen in PT. These deficits can lead to increased tendon strain due to reliance on passive rather than active structures. Weakness can also lead to focal strain in the tendon due to unequal force distribution.
Evidence supporting the role of foot posture and PT is conflicting. Studies have looked at arch height with PT and had varying results. One study concluded a more rigid foot associated with PT with volleyball players (64), and another study found a lower foot arch associated with subjects with unilateral and bilateral PT (65).
There has also been research into the motor control of the lower limb musculature in PT, showing increased corticospinal excitability particularly in the agonist (quadriceps), and increased cortical inhibition showing a change in the motor inhibition/excitability balance with PT (66).
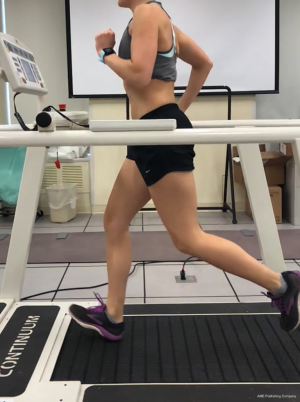
In terms of running mechanics and training, subjects with PT tend to land with a stiffer knee and may utilize a hip extension strategy rather than hip flexion with landing (67,68). Increased frequency and less experience can also predispose a runner to PT. One study showed, 5 to 8.5 consecutive days of running, as well as amateur runners running 20–50 km per week has increased reports of PT (69). Quadriceps overutilization eccentrically is associated with PT. An increase in peak ground reaction force in runners may lead to this muscular imbalance. This increase in peak ground reaction force (pGRF) may be caused by a lower step rate, overstriding (Figure 15), and increased vertical displacement (Figure 16). Less extensibility of quadriceps and hamstrings is seen commonly with PT, and may lead to decreased hip extension with push off in runners.
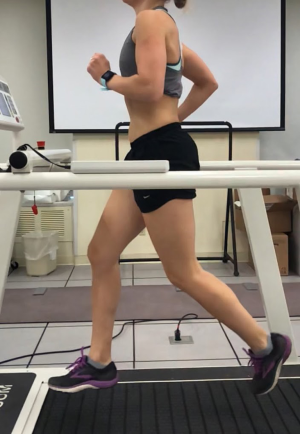
Treatment
Manual therapy
Due to the fact that tendinopathy is cyclical between an inflammatory and degenerative stage, manual therapy serves to restore normal healing in the involved tissue. To counteract this degeneration in the tendon, firm pressured cross friction massage in clinic and at home, or instrument assisted tissue mobilization is indicated, though this is not more beneficial than therapeutic exercise.
Muscle imbalances in the extensibility and tone of the quadriceps, hamstrings, and calf musculature should be addressed. Though in need of more research, muscle nociception from trigger points can create local pain in the knee joint, and neuroplastic changes in the central pain system. Modification of this input with TPDN may lead to less pain and increased motor activation of these muscles (70).
In theory, the tibiofemoral joint and patellofemoral joint arthrokinematics should be addressed and corrected as needed for pain relief. However, it is important to note manual therapy should be focused on efficient pain relief to the patient and should progress to therapeutic exercise as quickly as possible as it has the most research of improved outcomes with patients in PT.
Modalities
Extracorporeal shockwave therapy (ESWT)
ESWT has been used to treat chronic tendinopathies since the early 1990s. The mechanism of action of ESWT is not fully understood, and current research as to its efficacy in treating tendinopathies is conflicting. It is suspected that it can have both analgesic effects along with potential tissue regenerative effects. The efficacy of shockwave therapy is inconsistent, with some research finding no improvements (71). Other research is promising. One literature review by van Leeuwen et al. reviewed 7 articles utilizing ESWT in treating PT, demonstrated overall positive effects with pain management and knee function, however, several of the methodologies included were lacking and variable protocols were used (72). Another study looked at utilizing ESWT in athletes with a specific 16 week protocol and demonstrated decreased pain along with improved knee extensor strength and endurance (73). Finally, a meta-analysis by Liao et al. demonstrated improvements in not only pain, but also range of motion (74). Overall, extracorporeal shockwave is a safe, non-invasive therapy that can be used in conjunction with therapeutic exercise to improve pain and promote knee function.
Therapeutic ultrasound and phonophoresis/iontophoresis
Therapeutic ultrasound is another traditional modality used in the management of tendinopathies, however, it has limited to no research support for efficacy (75). Ultrasound has been shown to have positive effects on collagen production in vitro (76), however, these effects have not been studied in living organisms. A thermal effect can occur at certain dosage levels (77), however, it has not been proven that this thermal effect aids in healing or is an effective treatment of tendon inflammation.
Phonophoresis is a technique utilizing ultrasound to administer pharmaceutical agents such as corticosteroids or lidocaine to decrease pain. Iontophoresis is similar to phonophoresis except it utilizes direct current to administer the medication. Research has shown no added benefit of these agents and does not support the use of ultrasound, phonophoresis, or iontophoresis as a treatment modality for PT (78-80).
Infrapatellar strapping
Infrapatellar straps, commonly known as Cho-pat straps, are also used in the management of PT. These straps are used to alter load distribution on the patellar tendon and to decrease pain with activity. Infrapatellar strapping has been shown to decrease localized strain on the patellar tendon by increasing patella to patellar tendon angles and decreasing tendon length (81). This allows for increased force distribution with loading and decreased point of localized strain and irritation. One study examined the immediate effects on pain using infrapatellar strapping with jumping performance in young male athletes and demonstrated effective reduction in localized pain without reduced performance (82).
Infrapatellar strapping has also been shown to improve knee joint proprioception in those with decreased neuromuscular control. This improvement is especially evident in those with decreased duration of overall symptoms and smaller knee size (83). This increased proprioception can not only improve symptoms and knee function, it can also reduce potential re-injury risk.
Therapeutic exercise
Eccentric exercises have been strongly promoted to address patellar tendinopathies and achilles tendinopathies (84,85). However, a systematic review pointed to heavy slow resistance (HSR) training using a leg press, squats, and hack squats to have superior effects on tendon collagen turnover, subjective patient satisfaction, and equal VISA scores to eccentric training. A recent study in 2015 showed that isometric contractions as well can improve maximum voluntary isometric contraction (MVIC), reduce pain in patients with PT, and may be a secondary option to eccentric exercises (86). These training methods all show promise in treatment of PT, but further research is needed to look into HSR as only 2 moderate studies have been published.
Recent research has looked into not only strength training to improve the muscle-tendon complex strength, but also to focus on normalizing the motor control to restore normal movement patterns and decrease cortical inhibition of the muscle (87). This type of training termed tendon neuroplastic training, focuses on loading the tissues to restore tendon strength. It typically uses external pacing utilizing visual or auditory (metronome) cues that have shown to increase motor excitability in both lower extremities, as well as other areas in the body (88-91).
Treatment of PT involves education of biomechanics, frequency and rest with training. According to the American Orthopedic Society for Sport Medicine, a 10% maximum progression of frequency, intensity, or duration is acceptable for training. Certain patients may not be able to progress to heavy load training that occurs with HSR. A recent study showed that low load exercises with proper blood flow restriction, show a greater response in muscle strength as opposed to normal flow low load exercise. Blood flow restriction training involves strength training with the lower limb arterial blood flow occluded 80%. Blood flow restriction (BFR) also showed similar results to heavy training and may be an alternative to those having significant pain still with heavy load training. However, the goal is to progress to heavy lifting (92).
Neuromuscular re-education
Return to running should occur when patients are able to perform high impact single leg activities with no recurrent symptoms. Evidence has shown changes in motor control and activation in the quadriceps with PT (93). Assessment and treatment of compensations to balance the quadriceps to hip musculature activation is indicated. Running pattern should be assessed looking at vertical displacement under 2 inches and a 5–10% increase in step frequency if appropriate to reduce the pGRF (94). External cueing using visual feedback or auditory feedback for step rate and vertical displacement may be beneficial in the beginning of training and shoulder be phased out later on.
Bottom line
Running analysis
Runners with PT may present with an increased vertical displacement, abnormally low step frequency, or landing with a stiff, or straightened knee. Due to the pattern of frequent overutilization of the quadriceps in PT, runners may present with decreased hip extension.
Manual therapy
Degenerative tendon issues should be addressed by hands on cross friction or instrument assisted techniques (Figure 17). For musculature extensibility imbalances consider TPDN, instrument assisted, or hands on soft tissue mobilization. Joint arthrokinematic deficits should be efficiently addressed in the hip, tibiofemoral, patellofemoral, and talocrural joints.
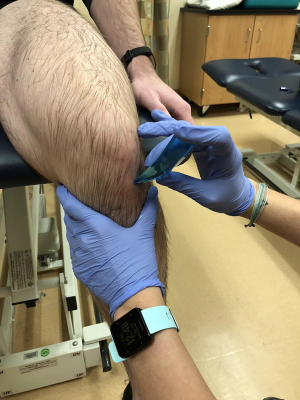
Modalities
Extracorporal shockwave can be effective in managing pain symptoms for acute PT but should not be used in isolation. Ultrasound, phonophoresis, and iontophoresis are not recommended modalities. Infrapatellar strapping can be useful in improving load distribution and proprioception to decrease pain with activity.
Therapeutic exercise
Malliaras et al. described a therapeutic exercise regimen for treating PT utilizing the previous most successful techniques studied in JOSPT (95).
Phase 1: quadriceps isometric training (Figure 18)
- Forty-five s holds at 5 repetitions with knee in midrange 2–3 X/day;
- Exercise should have an analgesic effect, and should be 70% of maximum force;
- When pain is not at a significant level for isotonic exercises, the patient can progress to phase 2. In this phase Blood flow restriction training to improve muscular strength may be beneficial up until the point of the patient being appropriate for HSR training.
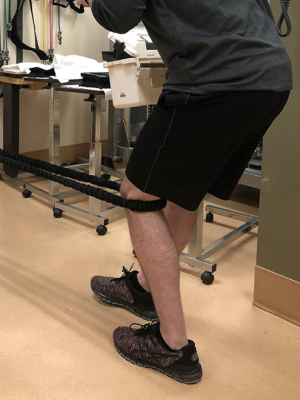
Phase 2: HSR training
- Performed 3–4 times per week, 15 reps with lower resistance progressing to 6 reps with higher resistance as tolerated utilizing leg press, hack squats, and squats using a full concentric and eccentric motion (Figure 19);
- Adjust the technique specifically for patients avoiding quadriceps activation on the affected side. Staggered stance or single leg squatting activities may be indicated for these patients. In the beginning use lower flexion angles as appropriate working towards 90 degrees flexion;
- External cues during this training is indicated, utilizing a metronome when the patient is comfortable (e.g., 3 s eccentric, 3 s concentric);
- When strength is comparable to the other side, light high impact activities are performed with minimal symptoms and return to baseline within the next day, the patient may begin high energy storage activities.

Phase 3: energy storage or plyometrics
- Begin with bilateral exercises focusing on take off and landing technique, jump height and rate of acceleration progression. Progression is dependent on the returning activity desired;
- Progress to single leg jumps, focus on power, speed of take off, and multi-directional changes. Tailor the treatment to functional movements the patient will perform when they return to activity.
Conclusions
PT is a chronic overuse degenerative condition beginning in the early ages of adolescence and adulthood. Assessment of this condition is extremely important to identify the impaired tissue at the inferior pole of the patella to rule out other patella-femoral issues. Quadriceps strength, posterior and anterior chain extensibility, and closed chain stability in squats, step downs, and other eccentric tasks should all be assessed. Jumping and running form should be assessed for a stiff knee landing position, and excessive vertical displacement in running.Treatment should focus on a progression of therapeutic exercise beginning with isometrics to build up strength and for pain relief. In this phase, manual therapy utilizing cross-friction massage and/or instrument assisted mobilization for the tendon, as well as manual soft tissue mobilization or TPDN to restore normal tone and extensibility in the muscle may be beneficial. Progression to HSR with a slow progression of higher reps with lower weight to heavier resistance and lower reps.External cues, such as using a metronome, may benefit the motor activation for the quadriceps during HSR and should be used. Blood flow restriction training may be utilized as a stepping stool for patients finding difficulty moving into heavier resistance to maintain strength gains in the muscle. Final review of technique for the symptom exacerbating activity is crucial for return to sport.
Discussion
Patellofemoral pain, ITBFS, and PT are the leading causes of knee pain in active adults, especially runners. Understanding the mechanism of these overuse injuries and how to best treat them allows for improved outcomes. These outcomes may result in decreased healthcare costs, avoidance of invasive procedures, and improved quality of life. With improved understanding and evidence informed treatment approaches, we can help keep our patient active and running.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van Gent RN, Siem D, van Middelkoop M, et al. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med 2007;41:469-80; discussion 480. [Crossref] [PubMed]
- Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med 2002;36:95-101. [Crossref] [PubMed]
- Mullaney MJ, Fukunaga T. Current concepts and treatment of patellofemoral compressive issues. Int J Sports Phys Ther 2016;11:891-902. [PubMed]
- Esculier JF, Bouyer LJ, Dubois B, et al. Effects of rehabilitation approaches for runners with patellofemoral pain: protocol of a randomised clinical trial addressing specific underlying mechanisms. BMC Musculoskelet Disord 2016;17:5. [Crossref] [PubMed]
- Thomeé R. A comprehensive treatment approach for patellofemoral pain syndrome in young women. Phys Ther 1997;77:1690-703. [Crossref] [PubMed]
- Fulkerson JP, Shea KP. Disorders of patellofemoral alignment. J Bone Joint Surg Am 1990;72:1424-9. [Crossref] [PubMed]
- Klingman RE, Liaos SM, Hardin KM. The effect of subtalar joint posting on patellar glide position in subjects with excessive rearfoot pronation. J Orthop Sports Phys Ther 1997;25:185-91. [Crossref] [PubMed]
- Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med 1991;19:131-7. [Crossref] [PubMed]
- Souza RB, Draper CE, Fredericson M, et al. Femur rotation and patellofemoral joint kinematics: a weight-bearing magnetic resonance imaging analysis. J Orthop Sports Phys Ther 2010;40:277-85. [Crossref] [PubMed]
- Duffey MJ, Martin DF, Cannon DW, et al. Etiologic factors associated with anterior knee pain in distance runners. Med Sci Sports Exerc 2000;32:1825-32. [Crossref] [PubMed]
- Dierks TA, Manal KT, Hamill J, et al. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther 2008;38:448-56. [Crossref] [PubMed]
- Ferber R, Kendall KD, Farr L. Changes in knee biomechanics after a hip-abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train 2011;46:142-9. [Crossref] [PubMed]
- Powers CM, Landel R, Perry J. Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain. Phys Ther 1996;76:946-55; discussion 956-67. [Crossref] [PubMed]
- Finnoff JT, Hall MM, Kyle K, et al. Hip strength and knee pain in high school runners: a prospective study. PM R 2011;3:792-801. [Crossref] [PubMed]
- Baldon Rde M, Nakagawa TH, Muniz TB, et al. Eccentric hip muscle function in females with and without patellofemoral pain syndrome. J Athl Train 2009;44:490-6. [Crossref] [PubMed]
- Tyler TF, Nicholas SJ, Mullaney MJ, et al. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med 2006;34:630-6. [Crossref] [PubMed]
- Smith AD, Stroud L, McQueen C. Flexibility and anterior knee pain in adolescent elite figure skaters. J Pediatr Orthop 1991;11:77-82. [Crossref] [PubMed]
- Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther 2005;35:793-801. [Crossref] [PubMed]
- Amis AA. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc Rev 2007;15:48-56. [Crossref] [PubMed]
- Whyte EF, Moran K, Shortt CP, et al. The influence of reduced hamstring length on patellofemoral joint stress during squatting in healthy male adults. Gait Posture 2010;31:47-51. [Crossref] [PubMed]
- Noehren B, Pohl MB, Sanchez Z, et al. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech (Bristol, Avon) 2012;27:366-71. [Crossref] [PubMed]
- Willy RW, Manal KT, Witvrouw EE, et al. Are mechanics different between male and female runners with patellofemoral pain? Med Sci Sports Exerc 2012;44:2165-71. [Crossref] [PubMed]
- Davis IS, Bowser BJ, Mullineaux DR. Greater vertical impact loading in female runners with medically diagnosed injuries: a prospective investigation. Br J Sports Med 2016;50:887-92. [Crossref] [PubMed]
- Collins NJ, Barton CJ, van Middelkoop M, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med 2018;52:1170-8. [Crossref] [PubMed]
- Jayaseelan DJ, Scalzitti DA, Palmer G, et al. The effects of joint mobilization on individuals with patellofemoral pain: a systematic review. Clin Rehabil 2018;32:722-33. [Crossref] [PubMed]
- Dommerholt J, Fernández-de-las-Peñas C. 1st edition. Trigger Point Dry Needling: An Evidence and Clinical-Based Approach. Churchill Livingstone, 2013.
- Sutlive TG, Golden A, King K, et al. Short-term effects of trigger point dry needling on pain and disability in subjects with patellofemoral pain syndrome. Int J Sports Phys Ther 2018;13:462-73. [Crossref] [PubMed]
- Aminaka N, Gribble PA. Patellar taping, patellofemoral pain syndrome, lower extremity kinematics, and dynamic postural control. J Athl Train 2008;43:21-8. [Crossref] [PubMed]
- Larsen B, Andreasen E, Urfer A, et al. Patellar taping: a radiographic examination of the medial glide technique. Am J Sports Med 1995;23:465-71. [Crossref] [PubMed]
- Gilleard W, McConnell J, Parsons D. The effect of patellar taping on the onset of vastus medialis obliquus and vastus lateralis muscle activity in persons with patellofemoral pain. Phys Ther 1998;78:25-32. [Crossref] [PubMed]
- Tiberio D. The effect of excessive subtalar joint pronation on patellofemoral mechanics: a theoretical model. J Orthop Sports Phys Ther 1987;9:160-5. [Crossref] [PubMed]
- Vicenzino B, Collins N, Crossley K, et al. Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: a randomised clinical trial. BMC Musculoskelet Disord 2008;9:27. [Crossref] [PubMed]
- Vicenzino B, Collins N, Cleland J, et al. A clinical prediction rule for identifying patients with patellofemoral pain who are likely to benefit from foot orthoses: a preliminary determination. Br J Sports Med 2010;44:862-6. [Crossref] [PubMed]
- Barton CJ, Menz HB, Crossley KM. Clinical predictors of foot orthoses efficacy in individuals with patellofemoral pain. Med Sci Sports Exerc 2011;43:1603-10. [Crossref] [PubMed]
- Barton CJ, Menz HB, Crossley KM. The immediate effects of foot orthoses on functional performance in individuals with patellofemoral pain syndrome. Br J Sports Med 2011;45:193-7. [Crossref] [PubMed]
- Smith TO, Bowyer D, Dixon J, et al. Can vastus medialis oblique be preferentially activated? A systematic review of electromyographic studies. Physiother Theory Pract 2009;25:69-98. [Crossref] [PubMed]
- Bhave A, Baker E. Prescribing quality patellofemoral rehabilitation before advocating operative care. Orthop Clin North Am 2008;39:275-85. v. [Crossref] [PubMed]
- Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Athl Train 2015;50:366-77. [Crossref] [PubMed]
- McHugh MP, Cosgrave CH. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports 2010;20:169-81. [PubMed]
- Vigotsky AD, Lehman GJ, Contreras B, et al. Acute effects of anterior thigh foam rolling on hip angle, knee angle, and rectus femoris length in the modified Thomas test. PeerJ 2015;3:e1281. [Crossref] [PubMed]
- MacDonald GZ, Penney MD, Mullaley ME, et al. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res 2013;27:812-21. [Crossref] [PubMed]
- Junker DH, Stöggl TL. The Foam Roll as a Tool to Improve Hamstring Flexibility. J Strength Cond Res 2015;29:3480-5. [Crossref] [PubMed]
- Willy RW, Scholz JP, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol, Avon) 2012;27:1045-51. [Crossref] [PubMed]
- Agresta C, Brown A. Gait Retraining for Injured and Healthy Runners Using Augmented Feedback: A Systematic Literature Review. J Orthop Sports Phys Ther 2015;45:576-84. [Crossref] [PubMed]
- van der Worp MP, van der Horst N, de Wijer A, et al. Iliotibial band syndrome in runners: a systematic review. Sports Med 2012;42:969-92. [Crossref] [PubMed]
- Ferber R, Noehren B, Hamill J, et al. Competitive female runners with a history of iliotibial band syndrome demonstrate atypical hip and knee kinematics. J Orthop Sports Phys Ther 2010;40:52-8. [Crossref] [PubMed]
- Nemeth WC, Sanders BL. The lateral synovial recess of the knee: anatomy and role in chronic Iliotibial band friction syndrome. Arthroscopy 1996;12:574-80. [Crossref] [PubMed]
- Fredericson M, Cookingham CL, Chaudhari AM, et al. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med 2000;10:169-75. [Crossref] [PubMed]
- Noble CA. Iliotibial band friction syndrome in runners. Am J Sports Med 1980;8:232-4. [Crossref] [PubMed]
- Noehren B, Davis I, Hamill J. ASB clinical biomechanics award winner 2006 prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin Biomech (Bristol, Avon) 2007;22:951-6. [Crossref] [PubMed]
- Hamill J, Miller R, Noehren B, et al. A prospective study of iliotibial band strain in runners. Clin Biomech (Bristol, Avon) 2008;23:1018-25. [Crossref] [PubMed]
- Tateuchi H, Shiratori S, Ichihashi N. The effect of angle and moment of the hip and knee joint on iliotibial band hardness. Gait Posture 2015;41:522-8. [Crossref] [PubMed]
- Hammer WI. The effect of mechanical load on degenerated soft tissue. J Bodyw Mov Ther 2008;12:246-56. [Crossref] [PubMed]
- Schwellnuss MP, Mackintosh L, Mee J. Deep Transverse Frictions in the Treatment of lliotibial Band Friction Syndrome in Athletes: A clinical trial. Physiotherapy 1992;78:564-8. [Crossref]
- Fredericson M, White JJ, Macmahon JM, et al. Quantitative analysis of the relative effectiveness of 3 iliotibial band stretches. Arch Phys Med Rehabil 2002;83:589-92. [Crossref] [PubMed]
- Schwellnus MP, Theunissen L, Noakes TD, et al. Anti-inflammatory and combined anti-inflammatory/analgesic medication in the early management of iliotibial band friction syndrome. A clinical trial. S Afr Med J 1991;79:602-6. [PubMed]
- Gunter P, Schwellnus MP. Local corticosteroid injection in iliotibial band friction syndrome in runners: a randomised controlled trial. Br J Sports Med 2004;38:269-72; discussion 272. [Crossref] [PubMed]
- Willy RW, Davis IS. The effect of a hip-strengthening program on mechanics during running and during a single-leg squat. J Orthop Sports Phys Ther 2011;41:625-32. [Crossref] [PubMed]
- Heiderscheit BC, Chumanov ES, Michalski MP, et al. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc 2011;43:296-302. [Crossref] [PubMed]
- Hafer JF, Brown AM, deMille P, et al. The effect of a cadence retraining protocol on running biomechanics and efficiency: a pilot study. J Sports Sci 2015;33:724-31. [Crossref] [PubMed]
- Magnusson SP, Narici MV, Maganaris CN, et al. Human tendon behaviour and adaptation, in vivo. J Physiol 2008;586:71-81. [Crossref] [PubMed]
- Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med 2009;43:409-16. [Crossref] [PubMed]
- Lian Ø, Scott A, Engebretsen L, et al. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med 2007;35:605-11. [Crossref] [PubMed]
- de Groot R, Malliaras P, Munteanu S, et al. Foot posture and patellar tendon pain among adult volleyball players. Clin J Sport Med 2012;22:157-9. [Crossref] [PubMed]
- Crossley KM, Thancanamootoo K, Metcalf BR, et al. Clinical features of patellar tendinopathy and their implications for rehabilitation. J Orthop Res 2007;25:1164-75. [Crossref] [PubMed]
- Goodwill AM, Pearce AJ, Kidgell DJ. Corticomotor plasticity following unilateral strength training. Muscle Nerve 2012;46:384-93. [Crossref] [PubMed]
- Edwards S, Steele JR, McGhee DE, et al. Landing strategies of athletes with an asymptomatic patellar tendon abnormality. Med Sci Sports Exerc 2010;42:2072-80. [Crossref] [PubMed]
- Bisseling RW, Hof AL, Bredeweg SW, et al. Relationship between landing strategy and patellar tendinopathy in volleyball. Br J Sports Med 2007;41:e8. [Crossref] [PubMed]
- Lopes AD, Hespanhol Júnior LC, Yeung SS, et al. What are the main running-related musculoskeletal injuries? A Systematic Review. Sports Med 2012;42:891-905. [Crossref] [PubMed]
- Dommerholt J. Dry needling - peripheral and central considerations. J Man Manip Ther 2011;19:223-7. [Crossref] [PubMed]
- Zwerver J, Hartgens F, Verhagen E, et al. No effect of extracorporeal shockwave therapy on patellar tendinopathy in jumping athletes during the competitive season: a randomized clinical trial. Am J Sports Med 2011;39:1191-9. [Crossref] [PubMed]
- van Leeuwen MT, Zwerver J, van den Akker-Scheek I. Extracorporeal shockwave therapy for patellar tendinopathy: a review of the literature. Br J Sports Med 2009;43:163-8. [Crossref] [PubMed]
- Cheng L, Chang S, Qian L, et al. Extracorporeal shock wave therapy for isokinetic muscle strength around the knee joint in athletes with patellar tendinopathy. J Sports Med Phys Fitness 2019;59:822-7. [Crossref] [PubMed]
- Liao CD, Xie GM, Tsauo JY, et al. Efficacy of extracorporeal shock wave therapy for knee tendinopathies and other soft tissue disorders: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2018;19:278. [Crossref] [PubMed]
- Warden SJ, Metcalf BR, Kiss ZS, et al. Low-intensity pulsed ultrasound for chronic patellar tendinopathy: a randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2008;47:467-71. [Crossref] [PubMed]
- Ramirez A, Schwane JA, McFarland C, et al. The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sports Exerc 1997;29:326-32. [Crossref] [PubMed]
- Chan AK, Myrer JW, Measom GJ, et al. Temperature changes in human patellar tendon in response to therapeutic ultrasound. J Athl Train 1998;33:130-5. [PubMed]
- Larsson ME, Käll I, Nilsson-Helander K. Treatment of patellar tendinopathy--a systematic review of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc 2012;20:1632-46. [Crossref] [PubMed]
- Klaiman MD, Shrader JA, Danoff JV, et al. Phonophoresis versus ultrasound in the treatment of common musculoskeletal conditions. Med Sci Sports Exerc 1998;30:1349-55. [PubMed]
- Penderghest CE, Kimura IF, Gulick DT. Double-blind clinical efficacy study of pulsed phonophoresis on perceived pain associated with symptomatic tendinitis. J Sport Rehabil 1998;7:9-19. [Crossref]
- Lavagnino M, Arnoczky SP, Dodds J, et al. Infrapatellar Straps Decrease Patellar Tendon Strain at the Site of the Jumper's Knee Lesion: A Computational Analysis Based on Radiographic Measurements. Sports Health 2011;3:296-302. [Crossref] [PubMed]
- Dar G, Mei-Dan E. Immediate effect of infrapatellar strap on pain and jump height in patellar tendinopathy among young athletes. Prosthet Orthot Int 2019;43:21-7. [Crossref] [PubMed]
- de Vries AJ, van den Akker-Scheek I, Haak SL, et al. Effect of a patellar strap on the joint position sense of the symptomatic knee in athletes with patellar tendinopathy. J Sci Med Sport 2017;20:986-91. [Crossref] [PubMed]
- Rutland M, O'Connell D, Brismée JM, et al. Evidence-supported rehabilitation of patellar tendinopathy. N Am J Sports Phys Ther 2010;5:166-78. [PubMed]
- Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes: a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med 2013;43:267-86. [Crossref] [PubMed]
- Rio E, Kidgell D, Purdam C, et al. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br J Sports Med 2015;49:1277-83. [Crossref] [PubMed]
- Weier AT, Pearce AJ, Kidgell DJ. Strength training reduces intracortical inhibition. Acta Physiol (Oxf) 2012;206:109-19. [Crossref] [PubMed]
- Rio E, Kidgell D, Moseley GL, et al. Tendon neuroplastic training: changing the way we think about tendon rehabilitation: a narrative review. Br J Sports Med 2016;50:209-15. [Crossref] [PubMed]
- Grau S, Maiwald C, Krauss I, et al. What are causes and treatment strategies for patellar-tendinopathy in female runners? J Biomech 2008;41:2042-6. [Crossref] [PubMed]
- Kidgell DJ, Stokes MA, Castricum TJ, et al. Neurophysiological responses after short-term strength training of the biceps brachii muscle. J Strength Cond Res 2010;24:3123-32. [Crossref] [PubMed]
- Leung M, Rantalainen T, Teo WP, et al. Motor cortex excitability is not differentially modulated following skill and strength training. Neuroscience 2015;305:99-108. [Crossref] [PubMed]
- Hughes L, Paton B, Rosenblatt B, et al. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med 2017;51:1003-11. [Crossref] [PubMed]
- Kidgell DJ, Pearce AJ. Corticospinal properties following short-term strength training of an intrinsic hand muscle. Hum Mov Sci 2010;29:631-41. [Crossref] [PubMed]
- Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health 2014;6:210-7. [Crossref] [PubMed]
- Malliaras P, Cook J, Purdam C, et al. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. J Orthop Sports Phys Ther 2015;45:887-98. [Crossref] [PubMed]


