
MR imaging for acute pancreatitis: the current status of clinical applications
Introduction
Acute pancreatitis (AP) is an acute chemical inflammation of the pancreas caused by activated pancreatin. The main clinical manifestations include abdomen pain, nausea and vomiting. Mild AP cases are mainly due to pancreatic edema and have a good prognosis, while severe AP cases are associated with hemorrhage, necrosis, infection, multiple organ failure and even shock (1). AP is usually caused by choledocholithiasis, hyperlipidemia and alcoholism. The disease course, clinical manifestations and prognosis vary greatly between individuals, a timely diagnosis and accurate evaluation of the disease severity is vital for clinical management (1). Furthermore, the complications of AP are closely related to the prognosis of the patients. The Acute Pancreatitis Classification Working Group modified the Atlanta Classification in 2012 (2). The 2012 revised Atlanta classification divided AP into interstitial edematous pancreatitis (IEP) and necrotizing pancreatitis (NP) and introduced two phases of the disease: early and late. The disease severity is classified as mild, moderately severe or severe (2). IEP refers to pancreatitis without necrosis in the pancreatic parenchyma and peripancreatic region, while NP involves necrosis in the pancreatic parenchyma or/and in any part of the peripancreatic region. In the 2012 revised Atlanta classification of AP, the course of the disease within 1 week is known as the early stage and after a week is known as the late stage. Regarding the severity classification, patients without organ failure are classified as having mild disease, patients with organ failure that recovered within 48 hours are classified as having moderately severe disease, and patients with organ failure that do not recover within 48 hours is classified as having severe disease (2). In addition, the new classification revised the terminology of local complications and subdivides the early complications into acute peripancreatic fluid collections (APFCs) and acute necrotic collections (ANCs) based on the necrotic debris. Delayed complications are also classified into pseudocysts and walled-off necrosis (WON) (3). The new classification clarifies physicians’ misconceptions about local complications in different specialties and provides a better background for combined multidisciplinary treatment.
CT is the first choice for diagnosing AP and its local complications (4). CT scans are fast and have high spatial resolution. CT scans can clearly display the pancreas and the surrounding tissues and overcome the limitations that ultrasound examinations are susceptible to, such as the influence of intestinal gas (5). However, CT has some shortcomings in evaluating the severity of AP. On enhanced CT scans, small nonenhanced areas may either be necrosis or local effusion in the pancreas, but it is difficult to distinguish them accurately by CT. Therefore, the possibility of overestimating the scope of necrosis may exist in the diagnosis of pancreatic necrosis with enhanced CT. Furthermore, focal adipose deposition in some elderly people may be misjudged as necrotic foci in the pancreas (6). Thus, the value of enhanced CT for small necrotic pancreases is limited, and the range of necrosis cannot be estimated correctly. As an alternate method for diagnosing acute pancreatitis, MR imaging (MRI) and MR cholangiopancreatography (MRCP) shows great potential in clinical applications (7). The benefits of MRI for acute pancreatitis are as follows: (I) the MRI sequence for T2-weighted image (T2WI) is highly sensitive to liquid and thus can visualize a small amount of liquid in mild pancreatitis, T2WI can characterize non-liquid materials in the pancreatic collections better than CT (6); (II) MRI clearly shows the areas of necrosis without enhancement and is safe for patients who are unable to receive iodinated contrast material because of allergies or kidney failure (8,9); (III) MRCP can noninvasively evaluate the changes of the bile ducts (especially the distal bile duct, which is difficult for ultrasound to show) and pancreatic duct system and help diagnose the etiology of the disease (10); (IV) recent studies have found that in addition to the air bubbles, high signals on the MRI diffusion-weighted imaging (DWI) sequences can also indicate the existence of infection to preliminarily determine whether the accumulation is infected (11); (V) novel MRI techniques such as intravoxel incoherent motion (IVIM) imaging can assess both perfusion and diffusion of the diseased tissues (12-14); (VI) MRI can be used to stage AP and clearly show local complications. It has been reported that non-enhanced MRI is more reliable and accurate than CT in estimating the severity of AP (9); and (VII) finally, MRI does not require ionizing radiation and does not lead to adverse effects on the human body.
In this article, we review the 2012 revised Atlanta classification of AP and recent advances in the clinical applications of MRI in acute pancreatitis by showing how MRI can provide more optimized information for clinical diagnosis and treatment plans.
MRI for the diagnosis of acute pancreatitis
Interstitial edematous pancreatitis
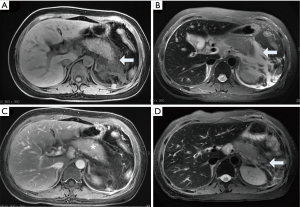
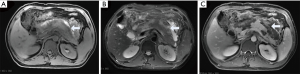
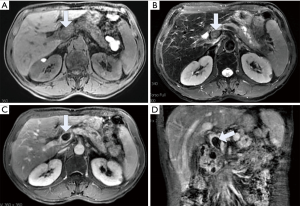
According to the 2012 revised Atlanta classification of AP, AP can be divided into interstitial edema pancreatitis and necrotizing pancreatitis (15). The former accounts for most AP cases and has a mild and self-limiting clinical course. The pancreas has diffuse or localized enlargement due to inflammatory edema, and this type of AP has no pancreatic necrosis or peripancreatic necrosis (2). The parenchyma of the pancreas may display normal or slight hypointensity on T1-weighted images (T1WI) and hyperintensity on T2WI. Patchy-like hyperintensity on T2WI could be observed in the peripancreatic region, perirenal space, and lesser omental bursa. There may be acute peripancreatic fluid collections. The pancreas shows homogeneous enhancement after an intravenous administration of a contrast agent such as Gd-DTPA (Figure 1).
Necrotizing pancreatitis
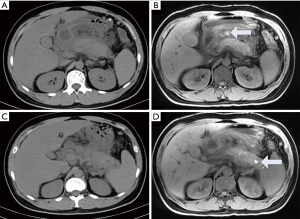
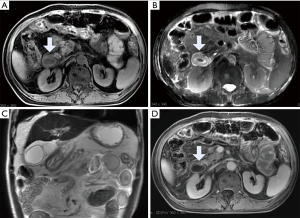
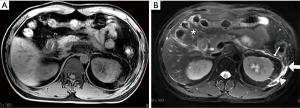
In necrotizing pancreatitis (NP), MRI has higher soft tissue contrast than CT and is superior to CT in showing hemorrhage and tissue necrosis. The sensitivity rate is approximately 76.5% (5). The necrotic area displays hypointensity on T1WI, hyperintensity on T2WI and no enhancement after an injection of contrast agent such as Gd-DTPA. NP can be divided into three subtypes based on the location of necrotic involvement: pancreatic parenchymal necrosis alone (PN), peripancreatic necrosis alone (PPN), and both pancreatic parenchymal necrosis and peripancreatic necrosis (3). The first type accounts for approximately 5% of all NP cases. The necrotic parenchymal area is not enhanced, and there is no necrotic area in the peripancreatic tissue (Figure 2). The extent of necrosis can be stratified as <30%, 30–50% and >50% to grade the severity of pancreatitis (3). The 2012 revised Atlanta classification of AP notes that since the evolution of pancreatic parenchymal necrosis often takes several days, it is uncertain whether the areas with reduced enhancement are necrosis if the enhanced scanning occurs during the first few days (3). To determine whether the early low enhancement area is necrotic, we can only observe whether the area eventually evolves into a completely nonenhanced area through follow-up, but the optimal follow-up time is not clear (Figure 3). When the pancreatic neck and body are totally necrotic, these signs typically indicate “disconnected pancreatic duct syndrome” (16).
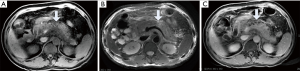
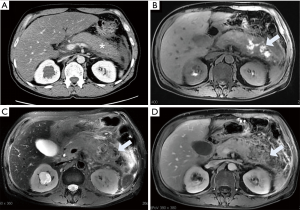
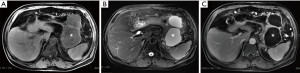
The second type can be seen in approximately 20% of necrotizing pancreatitis cases. Studies have suggested that the clinical course of PPN is milder than PN but more severe than IEP; therefore, PPN should be considered as a separate type different from the former two (17). The parenchyma of the pancreas shows a homogeneous signal on the T1WI and T2WI and is evenly enhanced (Figure 4). Nonfluid components can be found in the peripancreatic collections, which do not show enhancement after an administration of contrast agent. In most instances, the necrosis within the collections cannot be easily confirmed, which creates a difficulty in diagnosing this type of disease and means that PPN can only be diagnosed by fine needle aspiration. Since the morphological changes of extrapancreatic fat may only indicate the accumulation of fluid rather than fat necrosis, the accuracy of this definition in judging PPN demands further exploration. Meyrignac et al. (18) defined extrapancreatic fat infiltration, fluid accumulation or the mixed accumulation of liquid and solid components as PPN. Dellinger et al. (19) suggested that any accumulation with peripancreatic heterogeneity should be considered peripancreatic necrosis until confirmed. These two definitions may overestimate the incidence of PPN. Sternby et al. (20) found that different observers had poor consistency in the diagnosis of PPN, which indicated that the diagnosis of PPN was too subjective. There is no uniform standard for the quantitative analysis of PPN necrosis. A process to accurately identify the necrotic substances of PPN by objective methods still needs to be further studied in the future. Koutroumpakis et al. (21) described PPN as “limited“ and “diffuse“ in terms of the extent of PPN and the number of sites involved. A limited extent refers to an area of peripancreatic necrosis <5 cm, and diffuse extent refers to an extent of peripancreatic necrosis >5 cm or if >3 sites are involved. Rana et al. (17) also described PPN as “limited“ and “diffuse“, but in the literature “limited” extent refers to necrosis confined to the peripancreatic tissue, and the necrosis is “diffuse” if it extends to the paracolic sulcus or involves the pelvic cavity. Additionally, Meyrignac et al. (18) have reported that the PPN volume is correlated with the prognosis of AP, and a volume of PPN ≥100 mL may be more reliable than the current scoring systems in assessing organ failure, infection, length of stay and mortality.
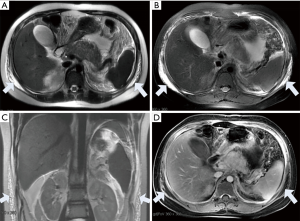
The last type is the most common type and accounts for approximately 75–80% of necrotizing pancreatitis (3). The necrosis in the pancreatic parenchyma is not enhanced, and the collections around the pancreas show mixed intensity on T1WI and T2WI but no enhancement after contrast injection (Figure 5). The complication and mortality rates of the last type are higher than those of the first two, and this type more likely requires surgical interventions than the other two types (22).
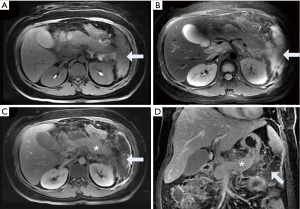
It should be noted that confirming necrosis is difficult at the early stage, even with enhanced scans. Early enhancement is likely to underestimate the final extent of necrosis. Low perfusion injury of the pancreas and the development of peripancreatic fat necrosis occurs after several days, and while hypoperfusion areas in prophase can be restored or can also deteriorate into necrosis (Figure 3). Therefore, the assessment of pancreatic necrosis is best performed 4 days after onset using enhanced MRI or CT (23). Moreover, the appearance of nonenhanced parenchyma after one week should be considered as necrosis (6) (Figure 3). Hemorrhage can demonstrate complex MRI signals. It is generally considered that the slightly high signal intensity on the T1WI suggests hemorrhage. MRI is superior to CT in visualizing hemorrhages, which can appear as iso-density on CT (24) (Figure 6). Necrotizing pancreatitis, especially the third type, has a high prevalence of co-occurring with bacterial infections. Infections are the second leading cause of death in patients with pancreatitis. As soon as the co-infection is identified, antibiotic therapy and surgical treatment (percutaneous drainage or open surgery) are necessary (1). Gas in the necrotic zone on the MRI may indicate infection (25).
MRI for local complications
Acute peripancreatic fluid collections (APFCs)
APFCs usually occur in IEP within 4 weeks of the onset of symptoms (26). APFCs lack cystic walls and solid components and are homogeneously hypointense on T1WIs and hyperintense on T2WI. These collections are limited in the peripancreatic fascial planes and are most commonly found in the lesser omental sac and left pararenal space (Figure 1) (3). APFCs are usually sterile, and if the acute edematous pancreatitis is cute, 50% of the collections can be spontaneously absorbed within 2–4 weeks (27). When the protracted course of APFCs is more than 4 weeks, they may develop into pseudocysts (28).
Pancreatic pseudocysts
After the occurrence of acute pancreatitis, pancreatic juice and various inflammatory exudates appear around the pancreas. If the collection is not absorbed after approximately four weeks, the collection may be surrounded by a capsule due to an inflammatory reaction, which forms a PPC. Because the cyst does not have a true epithelial tissue covering, it is called a pseudocyst. There are different studies on the risk factors of PPC in acute pancreatitis. Some researchers have found that the male sex, alcoholic pancreatitis and ascites are risk factors for PPC formation (29-31). PPCs larger than 6 cm in diameter may be associated with multiple complications, such as mass effect, infection, rupture and bleeding (3,32). In line with the 2012 revised Atlanta classification of AP, there is no non-liquid substance in pseudocysts. MRI can depict pancreatic pseudocysts to reveal a thin smooth wall, and the liquid content displays homogeneous intensity on T1WIs and T2WIs (Figure 7). Pancreatic juice constantly overflows from the ruptured pancreatic duct, leading to a gradual enlargement of the cysts and a long period of illness. Due to the need for surgical indications, it is important to visualize where the pancreatic duct breaks and its extent of rupture (33). T2WI, MRCP, and multiplanar reconstruction helps to visualize the broken pancreatic duct and its connection with pseudocysts. Pseudocyst infection is rare. When the pseudocyst is infected, the clear liquid becomes pus but still has no solid content, and the pseudocyst shows a slightly hyperintense signal on T2WIs and DWI scans. Studies have shown that patients who were only complicated with pseudocysts without infection or size-related symptoms should be treated with conservative treatment (34).
Acute necrotic collections (ANCs)
ANCs are only observed in necrotic pancreatitis within 4 weeks of onset and contain variable amounts of fluid and necrosis. Necrosis can involve the pancreatic parenchyma or/and peripancreatic tissues (Figure 5). Collections that are in the pancreatic parenchyma and around the necrotic pancreas are still termed ANCs (2,3). ANCs have no capsules. ANCs show mixed signals on T1WI and T2WI. On T2WIs, there can be flocculent low signal necrosis areas within the collections, which are not enhanced (Figure 5). It is very difficult to identify the ANCs and APFCs in the first week of onset, and the CT evaluation of ANC is subjective and cannot objectively judge whether there is necrotic tissue in the heterogeneous collections in peripancreatic tissue. Although MRI is more sensitive in differentiating different components in the accumulation of peripancreatic tissue, when the necrotic tissue fragments are small, MRI cannot determine whether the liquid that appears with high signal on the liquid-suppressed T2WI is entirely inflammatory fluid without doping necrotic cells, and thus, MRI still cannot accurately determine ANCs (35) (Figure 8). ANCs require drainage with a catheter to prevent infection and possible sepsis (36).
Walled-off necrosis (WON)
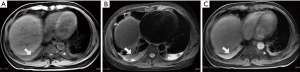
A severe and persistent clinical course or delayed deterioration of disease in necrotizing pancreatitis suggests the presence of WON, which is the maturation stage of ANCs (3). The most characteristic manifestation of WON on MRI is that the encapsulated effusion contains non-liquid substances, which are flocculent, with banded tissue fragments that are free and floating, and there is no enhanced signal on the enhanced scans. This non-liquid substance is the residue of the pancreas and extrapancreatic tissue, which are difficult to distinguish with CT while can be clearly visualized with MRI (5) (Figure 9). Patients with WON are commonly complicated with infection. Studies show that the mortality of patients with WON co-infection is higher than that of non-infected patients (37). Patients with sudden fevers, leukocytosis, sepsis or bubble signs, and gas-liquid interface on MRI suggest an infection (11) (Figure 10). When there is no characteristic imaging sign of infection but there is a clinical suspicion of co-infection, a fine needle aspiration biopsy is necessary for a definite diagnosis (11). Surgical treatment can be used only when WON is clinically diagnosed with an infection (infective necrosis) or to treat the increasing volume of WON or complications with bleeding, such as secondary pseudoaneurysms that cause obvious abdominal pain and distention (38).


Other complications
AP can involve the adjacent organs, such as the stomach, intestines, spleen, kidneys, and peripancreatic vessels. Changes of these organs and structures are reactions to inflammatory substances in the early stage. With the stabilization and improvements of the disease, these changes will gradually decrease or disappear. In the late stages, some changes may persist or even aggravate the complications of AP, such as including pseudoaneurysm of the peripancreatic vessels, venous thrombosis, spleen infarction, intestinal fistula.
Disconnected pancreatic duct syndrome (DPDS)
Severe necrotic pancreatitis with pancreatic duct disruption has a prevalence of approximately 10% to 31% (39). Persistent pancreatic fluid overflow causes various local complications, such as pancreatic pseudocysts, pancreatic ascites, pancreatic pleural fistulas, and pseudoaneurysms. ERCP is generally regarded as the gold standard for the diagnosis of pancreatic duct interruption. However, because ERCP is invasive, which is not suitable for acute pancreatitis (especially severe cases), and cannot show the pancreatic duct at the other end of the rupture, the evaluation of the main pancreatic duct interruption by ERCP is incomplete (40). The combination of MRI and MRCP provides a noninvasive method that can not only show the pancreas and peripancreatic changes but can also analyze the proximal and distal ends of the ruptured main pancreatic duct. Therefore, the use of MRI and MRCP is more advantageous to evaluate the main disconnected pancreatic duct syndrome caused by acute necrotizing pancreatitis (41). The visualization ratio of the pancreatic duct on MRCP is lower in acute pancreatitis than that in normal subjects, but the diameter of the main pancreatic duct is still within the normal range. The visualization of the pancreatic duct usually demands a combination of the coronal and axial SSFES T2WIs, axial FRFSE T2WI and MRCP image (42) (Figure 11). Studies have reported that the diagnosis of pancreatic duct disruption should be considered if the following occurs: (I) peripancreatic necrosis area at least 2 cm, and (II) MRCP shows that the main pancreatic duct of the upstream pancreatic tissue travels to the WON area of the intra and/or extrapancreatic tissue and approaches with a right angle into the fluid or necrotic tissue (33). Our previous study showed that as the severity increases, the incidence of pancreatic duct rupture also increases. The development of a disruption and a ruptured pancreatic duct on the MRI scan can be another credible indicator of the severity of acute pancreatitis (43), and the severity determines the clinical treatment method and predicts the short-term and long-term complications.

Hemorrhage and peripheral vascular invasion
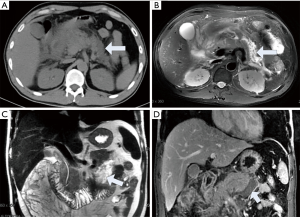
Hemorrhage in acute pancreatitis mainly includes gastrointestinal bleeding, intra-abdominal blood accumulation and bleeding in the pseudocysts. The main mechanism is that the tissue-dissolving enzymes released by the pancreas can corrode the wall of the peripancreatic vessels, which causes chemical inflammation of the vascular wall, formation of venous thrombosis, and massive hemorrhage due to the destruction of the blood vessel wall (44). Hemorrhages on CT show a slightly high density (>35 HU). Over time, the density gradually decreases. Compared with CT, MRI is more sensitive to visualizing hemorrhages, which are hyperintense on T1WI during acute phase and have signals that persist longer than on CT images (24) (Figures 3,6).
Involvement of the peripancreatic vessels, which includes a series of vascular abnormalities, is common in AP and especially in NP; the prevalence is approximately 16.9% (45). The pathogenesis is probably direct exudative damage to the pancreatic vessels. Arteritis and pseudoaneurysm occur in arterial involvement; phlebitis, venous thrombosis, and pancreatic portal hypertension/portal vein cavernous changes occur in venous involvement (46). The main MRI manifestations are loss of normal flow in the lumen, and the signal void on the T1WI and the T2WI is replaced by local hyperintensity. The enhanced scan shows inhomogeneous enhancement and segmental poor enhancement in the affected segments (Figure 12). As the acute pancreatitis worsens, the vascular complications become more common. A study showed that for splenic vein thrombosis and mesenteric venous thrombosis, the occurrence of splenic artery and vein inflammation was positively correlated with the MRSI scores.
Gastrointestinal tract involvement
Studies show that approximately 63% of patients with AP have gastrointestinal abnormalities (47). The abnormalities are characterized by gastrointestinal dilatation, mild thickening of the gastrointestinal wall, stratification of the bowel wall during its enhancement on the arterial phase after an intravenous injection of a contrast agent. The thickening of the intestine wall is uniform, which is different from the heterogeneous and eccentric thickening of neoplastic lesions. The middle wall shows high signals, and the internal and external ring show hypointensity; together, these form the so-called “target sign” on T2WI, and the post-contrast scan also shows stratified enhancement (48) (Figure 13). Ji et al. (47) hypothesized that the closer to the pancreas, the more serious the abnormalities in the tissues and organs; they found that the colon and duodenum were the most vulnerable organs. Gastrointestinal tract involvement plays an important role in assessing the severity and monitoring the efficacy of treatment (47). If attention is not paid to intestinal injuries in the early stage of the disease, with the progression of the disease a large number of toxins and inflammatory mediators can be released, and bacteria can even be translocated. Once “intestinal infection” occurs, there may be fatal consequences. Therefore, clinicians should actively observe changes in the gastrointestinal tract during the course of disease with MRI, and adjust the therapy according to the recovery of the gastrointestinal tract.
Mesenteric and retroperitoneal fascia space changes
AP can involve the mesentery and manifest as the spread of inflammation from the root of the mesentery to the mesentery along with transverse-mesocolon edema and effusions (49). Mesenteric edema and effusion are characterized by stripes and small slices of hyperintensity on T2WI (Figure 14). The changes in the blood vessels include wall thickening, rough texture, enlarged lumen and collateral circulation. Studies have shown that small intestinal mesentery and transverse mesocolic invasion are common in AP, which are positively correlated with a high MRSI score, and both of these are aggravated with increases in the severity of AP (50).
The pancreas is located in the retroperitoneal anterior pararenal space, so the inflammatory pancreatic juice of AP can penetrate into the anterior pararenal space and can also spread into the perirenal space, the posterior pararenal space, the abdominal cavity, and even the subphrenic and pelvic cavities (51,52). In addition to the above-mentioned retroperitoneal space, Molmenti et al. (53) proposed a new anatomical concept for the structure of the retroperitoneal space, the interfascial plane. The retroperitoneal space is composed of several discontinuous layers and potential interfascial planes. Interfascial plane involvement is a common MRI manifestation of acute pancreatitis on both sides, but the rate of left side involvement is significantly higher than that of the right side (51). MRI can accurately depict the extent of interfascial plane involvement in pancreatitis of different severities and infer the route of inflammation transmission through the sequence of fascial involvement (Figure 14) (50,51).
Complications of the liver and biliary tract system
AP leads to abnormal peripancreatic microcirculation, systemic inflammatory response syndrome (SIRS) and bile duct obstructions, which may lead to abnormal liver changes (54). Routine MRI findings of AP with liver abnormalities include hepatic steatosis, transient hepatic perfusion abnormalities, periportal lymphatic stasis and perihepatic effusion (Figure 15). These abnormalities can reflect different degrees of liver injury, and the incidence of hepatic steatosis is the highest out of the incidences of all abnormalities (55). Liver injuries caused by AP can not only aggravate the severity of pancreatitis but can also develop into liver failure, which results in the rapid death of the patient. The fatty liver finding on MRI is positively correlated with the triglyceride level, and fatty liver can also be alleviated with the improvement of pancreatitis (56). As the lower part of the common bile duct travels in the head of the pancreas, most of the diseases of the head of the pancreas can cause the narrowing and obstruction of the lumen of the common bile duct. Early decompression of the biliary tract system can prevent progression of the disease and avoid recurrent pancreatitis (57).

Others
AP can also involve the urinary system, respiratory system, bone and skin. Acute renal failure is an early complication of severe AP and has a high mortality rate. The incidence of organ failure in other systems increases significantly after acute renal failure (58). There is more perirenal involvement in AP on MRI than renal parenchyma involvement, and the morbidity of perirenal involvement has been noted to be positively correlated with a high MRSI score (51). MRI findings of abnormal changes in the renal and perirenal space include abnormal perfusion of the renal parenchyma, swelling of perirenal fat, fluid collections in the perirenal space, renal venous thrombosis, etc. (51). Respiratory complications are very common in patients with acute pancreatitis. Hypoxemia can be the only manifestation in mild cases, and respiratory failure can occur in severe cases (59). Studies have shown that abdominal hypertension and systemic inflammatory response syndrome associated with acute pancreatitis are the main causes of early lung injury. Abdominal hypertension can lead to increased intrathoracic pressure, pleural effusion, atelectasis, pneumonia, airway trapping and other pulmonary complications; all of these can then lead to abnormal gas exchange, which is one of the most common causes of early lung injury in acute pancreatitis (60,61). In addition, systemic inflammatory response is also an important mechanism that leads to lung injury in acute pancreatitis. In acute pancreatitis, vasoactive substances and cytokines are released into the blood flow and into the lung tissue, which can lead to endothelial damage and capillary leakage; additionally, a large number of protein-rich exudates are released into the alveolar space, leading to interstitial pulmonary edema (59). MRI shows diffuse pulmonary exudative patches, bilateral lobular consolidation and pleural effusions (62) (Figure 16). Injuries of the bone and skin secondary to pancreatitis may be caused by high pancreatic lipase in the blood. On MRI, these injuries can be characterized by inflammation of the joints, focal bone destruction, and abdominal wall edema (Figure 17). Furthermore, a few patients may have pancreatic ascites, that is, pancreatic juice that directly or indirectly enters the peritoneal cavity after pancreatic duct rupture. Physicians should treat these injuries as early as possible to reduce mortality (63). In acute pancreatitis, the spleen is also a commonly involved organ; common splenic abnormalities are splenomegaly and splenic infarction. In addition to routine sequences, quantitative analysis of the spleen with IVIM sequences might be useful for visualizing splenic perfusion changes in AP (14).
MRI for the severity of acute pancreatitis
The MRSI derived from the CT severity index (CTSI) is used to assess the severity of AP by combining inflammation around the pancreas and necrosis of the pancreas parenchyma to evaluate the local conditions, and the MRSI is comparable to the CTSI in evaluating the severity of AP (5). It has been shown that the MRSI is superior to the Acute Physiology and Chronic Health Evaluation II (APACHEII) in assessing local complications from pancreatitis but has a limited role in determining systemic complications, where the APACHE II score excels (64).
Certain imaging signs beyond the MRSI scoring system can also indicate the severity of AP. Studies have shown that MRI can display positive correlations between the time of improvement of the gastrointestinal tract abnormalities and the time of hospitalization, the disappearance time of abdominal pain and abdominal distention, and the normal time of amylase recovery and the recovery time of the diet (47). MRI can also show peripancreatic vascular changes, and the severity of vascular involvement is positively correlated with the severity of AP (14). In addition, the incidence of liver and kidney dysfunction, pulmonary inflammation, perirenal space involvement, interfascial plane involvement and abdominal wall edema increases with the severity of AP (50-52). It has been reported that the morphological changes of the main pancreatic duct (flexion degree and curve length) are related to AP occurrence. The greater the flexion of the main pancreatic duct, the greater the possibility of the occurrence of AP. The incidence of pancreatic duct rupture increases with the severity of AP and can be used as another simple auxiliary index to assess and predict the severity of AP (43). In terms of clinical treatment, surgical indications and the timing of the surgery are important for patients who require surgical intervention. MRI can help surgical planning by demonstrating the scope of necrotic tissue, the increase or decrease in the amount of peripancreatic retroperitoneal fluid, and the clear demarcation of the necrotic tissue and the normal tissue.
In recent years, DWI, ADC values and intravoxel incoherent motion (IVIM) have been applied in the abdomen, all objectively reflect the movement of water molecules in tissues and their biological behavior (65). During AP, there is the presence of swollen pancreatic acinar cells, interstitial congestion, edema and inflammatory cell infiltration (66). de Freitas Tertulino et al. (65) found that the ADC value can help to distinguish mild inflammation from necrosis of the pancreas, which is significant for evaluating the severity of acute pancreatitis. Kovalska et al. (66) suggested that microcirculatory disturbances can cause ischemia, reperfusion injury, and even pancreatic tissue damage. The severity of AP actually hinges on the severity of microcirculation disturbance. MR perfusion imaging can reflect the changes in early pancreatic blood flow, which can diagnose pancreatic necrosis in the early stage of disease by quantitatively analyzing pancreatic blood perfusion (67). Furthermore, Hu et al. showed that evaluation of pancreatic perfusion with DCE-MRI was helpful in grading AP severity. They found that pancreatic perfusion decreased with the aggravation of AP (68). Sahani et al. (69) used animal models to confirm the feasibility and accuracy of computed tomography perfusion in detecting pancreatic necrosis. In the necrotic area, perfusion parameters such as blood flow, blood volume and surface permeability were significantly reduced, but these parameters did not change significantly in the edema area. Moreover, diffusion tensor imaging (DTI) has the capability to detect changes in the degree of diffusion anisotropy and molecular diffusion in AP patients by calculating the FA and ADC values of the pancreas to evaluate the severity of AP (70).
Conclusions
In summary, MRI has advantages in the diagnosis of AP and its complications. The severity of AP can be estimated by combining the morphological changes of the pancreas, peripancreatic tissues and other organs on MR images. The rapid development of functional imaging techniques based on MRI has greatly increased the possibility of radiologists accurately evaluating the severity and progression of acute pancreatitis.
Acknowledgments
Funding: This study is supported by national nature science foundation of China (No. 81871440).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2016;375:1972-81. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012;262:751-64. [Crossref] [PubMed]
- Ju S, Chen F, Liu S, et al. Value of CT and clinical criteria in assessment of patients with acute pancreatitis. Eur J Radiol 2006;57:102-7. [Crossref] [PubMed]
- Arvanitakis M, Delhaye M, De Maertelaere V, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 2004;126:715-23. [Crossref] [PubMed]
- Kamal A, Singh VK, Akshintala VS, et al. CT and MRI assessment of symptomatic organized pancreatic fluid collections and pancreatic duct disruption: an interreader variability study using the revised Atlanta classification 2012. Abdom Imaging 2015;40:1608-16. [Crossref] [PubMed]
- O'Neill E, Hammond N, Miller FH. MR imaging of the pancreas. Radiol Clin North Am 2014;52:757-77. [Crossref] [PubMed]
- Wang YX, Chen S, Morcos SK. Contrast-enhanced CT in acute pancreatitis. Br J Radiol 1999;72:1029. [Crossref] [PubMed]
- Viremouneix L, Monneuse O, Gautier G, et al. Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging 2007;26:331-8. [Crossref] [PubMed]
- Barlow AD, Haqq J, McCormack D, et al. The role of magnetic resonance cholangiopancreatography in the management of acute gallstone pancreatitis. Ann R Coll Surg Engl 2013;95:503-6. [Crossref] [PubMed]
- Islim F, Salik AE, Bayramoglu S, et al. Non-invasive detection of infection in acute pancreatic and acute necrotic collections with diffusion-weighted magnetic resonance imaging: preliminary findings. Abdom Imaging 2014;39:472-81. [Crossref] [PubMed]
- Li YT, Cercueil JP, Yuan J, et al. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant Imaging Med Surg 2017;7:59-78. [Crossref] [PubMed]
- Ma W, Zhang G, Ren J, et al. Quantitative parameters of intravoxel incoherent motion diffusion weighted imaging (IVIM-DWI): potential application in predicting pathological grades of pancreatic ductal adenocarcinoma. Quant Imaging Med Surg 2018;8:301-10. [Crossref] [PubMed]
- Xie CL, Zhang M, Chen Y, et al. Spleen and splenic vascular involvement in acute pancreatitis: an MRI study. Quant Imaging Med Surg 2018;8:291-300. [Crossref] [PubMed]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586-90. [Crossref] [PubMed]
- Pelaez-Luna M, Vege SS, Petersen BT, et al. Disconnected pancreatic duct syndrome in severe acute pancreatitis: clinical and imaging characteristics and outcomes in a cohort of 31 cases. Gastrointest Endosc 2008;68:91-7. [Crossref] [PubMed]
- Rana SS, Sharma V, Sharma RK, et al. Clinical significance of presence and extent of extrapancreatic necrosis in acute pancreatitis. J Gastroenterol Hepatol 2015;30:794-8. [Crossref] [PubMed]
- Meyrignac O, Lagarde S, Bournet B, et al. Acute Pancreatitis: Extrapancreatic Necrosis Volume as Early Predictor of Severity. Radiology 2015;276:119-28. [Crossref] [PubMed]
- Dellinger EP, Forsmark CE, Layer P, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 2012;256:875-80. [Crossref] [PubMed]
- Sternby H, Verdonk RC, Aguilar G, et al. Significant inter-observer variation in the diagnosis of extrapancreatic necrosis and type of pancreatic collections in acute pancreatitis - An international multicenter evaluation of the revised Atlanta classification. Pancreatology 2016;16:791-7. [Crossref] [PubMed]
- Koutroumpakis E, Dasyam AK, Furlan A, et al. Isolated Peripancreatic Necrosis in Acute Pancreatitis Is Infrequent and Leads to Severe Clinical Course Only When Extensive: A Prospective Study From a US Tertiary Center. J Clin Gastroenterol 2016;50:589-95. [Crossref] [PubMed]
- Kleespies A, Thasler WE, Schäfer C, et al. Acute pancreatitis: is there a need for surgery? Z Gastroenterol 2008;46:790-8. [Crossref] [PubMed]
- Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331-6. [Crossref] [PubMed]
- Tang MY, Chen TW, Bollen TL, et al. MR imaging of hemorrhage associated with acute pancreatitis. Pancreatology 2018;18:363-9. [Crossref] [PubMed]
- Türkvatan A, Erden A, Türkoğlu MA, et al. Imaging of acute pancreatitis and its complications. Part 2: complications of acute pancreatitis. Diagn Interv Imaging 2015;96:161-9. [Crossref] [PubMed]
- Sheu Y, Furlan A, Almusa O, et al. The revised Atlanta classification for acute pancreatitis: a CT imaging guide for radiologists. Emerg Radiol 2012;19:237-43. [Crossref] [PubMed]
- Ahmed A, Gibreel W, Sarr MG. Recognition and Importance of New Definitions of Peripancreatic Fluid Collections in Managing Patients with Acute Pancreatitis. Dig Surg 2016;33:259-66. [Crossref] [PubMed]
- Bouwense SA, van Brunschot S, van Santvoort HC, et al. Describing Peripancreatic Collections According to the Revised Atlanta Classification of Acute Pancreatitis: An International Interobserver Agreement Study. Pancreas 2017;46:850-7. [Crossref] [PubMed]
- Kim YK, Ko SW, Kim CS, et al. Effectiveness of MR imaging for diagnosing the mild forms of acute pancreatitis: comparison with MDCT. J Magn Reson Imaging 2006;24:1342-9. [Crossref] [PubMed]
- Poornachandra KS, Bhasin DK, Nagi B, et al. Clinical, biochemical, and radiologic parameters at admission predicting formation of a pseudocyst in acute pancreatitis. J Clin Gastroenterol 2011;45:159-63. [Crossref] [PubMed]
- Kim KO, Kim TN. Acute pancreatic pseudocyst: incidence, risk factors, and clinical outcomes. Pancreas 2012;41:577-81. [Crossref] [PubMed]
- Türkvatan A, Erden A, Seçil M, et al. Fluid collections associated with acute pancreatitis: a pictorial essay. Can Assoc Radiol J 2014;65:260-6. [Crossref] [PubMed]
- Sandrasegaran K, Tann M, Jennings SG, et al. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics 2007;27:1389-400. [Crossref] [PubMed]
- van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141:1254-63. [Crossref] [PubMed]
- Dhaka N, Samanta J, Kochhar S, et al. Pancreatic fluid collections: What is the ideal imaging technique. World J Gastroenterol 2015;21:13403-10. [Crossref] [PubMed]
- Manrai M, Kochhar R, Gupta V, et al. Outcome of Acute Pancreatic and Peripancreatic Collections Occurring in Patients With Acute Pancreatitis. Ann Surg 2018;267:357-63. [Crossref] [PubMed]
- Hughey M, Taffel M, Zeman RK, et al. The diagnostic challenge of the sequelae of acute pancreatitis on CT imaging: a pictorial essay. Abdom Radiol (NY) 2017;42:1199-209. [Crossref] [PubMed]
- Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400-15; 1416.
- Uomo G, Molino D, Visconti M, et al. The incidence of main pancreatic duct disruption in severe biliary pancreatitis. Am J Surg 1998;176:49-52. [Crossref] [PubMed]
- Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol 2006;186:499-506. [Crossref] [PubMed]
- Xiao B, Zhang XM, Tang W, et al. Magnetic resonance imaging for local complications of acute pancreatitis: a pictorial review. World J Gastroenterol 2010;16:2735-42. [Crossref] [PubMed]
- Larena JA, Astigarraga E, Saralegui I, et al. Magnetic resonance cholangiopancreatography in the evaluation of pancreatic duct pathology. Br J Radiol 1998;71:1100-4. [Crossref] [PubMed]
- Peng R, Zhang XM, Ji YF, et al. Pancreatic duct patterns in acute pancreatitis: a MRI study. PLoS One 2013;8:e72792. [Crossref] [PubMed]
- Shen X, Sun J, Zhang J, et al. Risk Factors and Outcome for Massive Intra-Abdominal Bleeding Among Patients With Infected Necrotizing Pancreatitis. Medicine (Baltimore) 2015;94:e1172. [Crossref] [PubMed]
- Mortelé KJ, Mergo PJ, Taylor HM, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol 2004;52:67-72. [Crossref] [PubMed]
- Charvat F, Maskova J, Belina F, et al. Portal vein erosion: a rare hemorrhagic complication of acute pancreatitis treated by percutaneous stent-graft placement. J Vasc Interv Radiol 2010;21:411-2. [Crossref] [PubMed]
- Ji YF, Zhang XM, Mitchell DG, et al. Gastrointestinal tract involvement in acute pancreatitis: initial findings and follow-up by magnetic resonance imaging. Quant Imaging Med Surg 2017;7:641-53. [Crossref] [PubMed]
- Tolan DJ, Greenhalgh R, Zealley IA, et al. MR enterographic manifestations of small bowel Crohn disease. Radiographics 2010;30:367-84. [Crossref] [PubMed]
- Mindelzun RE, Jeffrey RB, Lane MJ, et al. The misty mesentery on CT: differential diagnosis. AJR Am J Roentgenol 1996;167:61-5. [Crossref] [PubMed]
- Chi XX, Zhang XM, Chen TW, et al. The normal transverse mesocolon and involvement of the mesocolon in acute pancreatitis: an MRI study. PLoS One 2014;9:e93687. [Crossref] [PubMed]
- Chi XX, Chen TW, Huang XH, et al. Magnetic resonance imaging of retroperitoneal interfascial plane involvement in acute pancreatitis. Quant Imaging Med Surg 2016;6:250-8. [Crossref] [PubMed]
- Yang R, Jing ZL, Zhang XM, et al. MR imaging of acute pancreatitis: correlation of abdominal wall edema with severity scores. Eur J Radiol 2012;81:3041-7. [Crossref] [PubMed]
- Molmenti EP, Balfe DM, Kanterman RY, et al. Anatomy of the retroperitoneum: observations of the distribution of pathologic fluid collections. Radiology 1996;200:95-103. [Crossref] [PubMed]
- Kelly DM, McEntee GP, McGeeney KF, et al. Microvasculature of the pancreas, liver, and kidney in cerulein-induced pancreatitis. Arch Surg 1993;128:293-5. [Crossref] [PubMed]
- Xu C, Qiao Z, Lu Y, et al. Influence of Fatty Liver on the Severity and Clinical Outcome in Acute Pancreatitis. PLoS One 2015;10:e0142278. [Crossref] [PubMed]
- Xiao B, Zhang XM, Jiang ZQ, et al. Fatty liver in acute pancreatitis: characteristics in magnetic resonance imaging. J Comput Assist Tomogr 2012;36:400-5. [Crossref] [PubMed]
- Pezzilli R, Billi P, Barakat B, et al. Effects of early ductal decompression in human biliary acute pancreatitis. Pancreas 1998;16:165-8. [Crossref] [PubMed]
- Naqvi R. Acute Kidney Injury in association with Acute Pancreatitis. Pak J Med Sci 2018;34:606-9. [Crossref] [PubMed]
- Dombernowsky T, Kristensen MØ, Rysgaard S, et al. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreatitis. Pancreatology 2016;16:756-60. [Crossref] [PubMed]
- Ke L, Tong ZH, Ni HB, et al. The effect of intra-abdominal hypertension incorporating severe acute pancreatitis in a porcine model. PLoS One 2012;7:e33125. [Crossref] [PubMed]
- Landahl P, Ansari D, Andersson R. Severe Acute Pancreatitis: Gut Barrier Failure, Systemic Inflammatory Response, Acute Lung Injury, and the Role of the Mesenteric Lymph. Surg Infect (Larchmt) 2015;16:651-6. [Crossref] [PubMed]
- Elder AS, Saccone GT, Dixon DL. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology 2012;12:49-56. [Crossref] [PubMed]
- Lipsett PA, Cameron JL. Internal pancreatic fistula. Am J Surg 1992;163:216-20. [Crossref] [PubMed]
- Tang W, Zhang XM, Xiao B, et al. Magnetic resonance imaging versus Acute Physiology And Chronic Healthy Evaluation II score in predicting the severity of acute pancreatitis. Eur J Radiol 2011;80:637-42. [Crossref] [PubMed]
- de Freitas Tertulino F, Schraibman V, Ardengh JC, et al. Diffusion-weighted magnetic resonance imaging indicates the severity of acute pancreatitis. Abdom Imaging 2015;40:265-71. [Crossref] [PubMed]
- Kovalska I, Dronov O, Zemskov S, et al. Patterns of pathomorphological changes in acute necrotizing pancreatitis. Int J Inflam 2012;2012:508915. [Crossref] [PubMed]
- Bize PE, Platon A, Becker CD, et al. Perfusion measurement in acute pancreatitis using dynamic perfusion MDCT. AJR Am J Roentgenol 2006;186:114-8. [Crossref] [PubMed]
- Hu R, Yang H, Chen Y, et al. Dynamic Contrast-Enhanced MRI for Measuring Pancreatic Perfusion in Acute Pancreatitis: A Preliminary Study. Acad Radiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Sahani DV, Holalkere NS, Kambadakone A, et al. Role of computed tomography perfusion in the evaluation of pancreatic necrosis and pancreatitis after endoscopic ultrasound-guided ablation of the pancreas in a porcine model. Pancreas 2009;38:775-81. [Crossref] [PubMed]
- Li X, Zhuang L, Zhang X, et al. Preliminary Study of MR Diffusion Tensor Imaging of Pancreas for the Diagnosis of Acute Pancreatitis. PLoS One 2016;11:e0160115. [Crossref] [PubMed]


