
Prognostic significance of the PANK family expression in acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is a hematological malignancy due to unchecked clonal expansion of mutated myeloid hematopoietic cells (1). It is highly aggressive and harbors widely heterogeneous clinical and genetic features, which makes the individualized diagnosis, treatment and prognostication of each patient particularly challenging (2). The current AML risk stratification systems are not perfect, although many genetic markers have been included to assist the prognosis evaluation, such as FLT3-ITD, NMP1, TP53 and CEBPA, of which FLT3-ITD and TP53 mutations are adverse, and CEBPA double-mutations and NPM1 mutation are favorable prognostic markers in AML (3-6). Nevertheless, with the development of next generation sequencing (NGS), the AML mutational spectrum has gained more details, more genetic and epigenetic markers are being identified for better risk stratification and therapeutic design (7,8).
Pantothenate kinase (PANK) is a rate-limiting enzyme in coenzyme A (CoA) synthesis (9). It has four isoforms encoded by PANK1-4, but only the proteins encoded by PANK1-3 have functional pantothenate kinase activities (10). Previous studies showed that the PANKs are involved in tumorigenesis. PANK2 has positive effect on the treatment of papillary and anaplastic thyroid cancer by triggering larger transcriptomic alterations (11). MiR-103, which is buried in the PANK3 gene, is a useful prognostic biomarker for leukemia (12). PANK1 plays an important role in the regulation of p53-dependent energy homeostasis (13). The prognostic role of the PANK family in AML remains unknown. Here we aim to analyze the prognostic significance of the expression of the PANK family in AML.
Methods
Patients
The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/) was screened for AML patients with complete PANK1-4 expression data and 84 patients who met the criteria were included in the study (14). All patients were between age 22 and 88, and only received chemotherapy, i.e., no patient had stem cell transplant. Data on the patients’ clinical and molecular characteristics at diagnosis were downloaded from the database, including peripheral blood (PB) white blood cell (WBC) counts, blast percentages in PB and bone marrow (BM), the distributions of the French-American-British (FAB) subtypes and the molecular-cytogenetic risk group, and the mutation frequencies of common recurrent genetic mutations. Event-free survival (EFS) and overall survival (OS) were study endpoints. Informed consents were obtained from all patients, and the study protocol was approved by the Human Research Council of University of Washington.
Statistical analysis
The clinical and molecular characteristics of patients were summarized using descriptive statistics. Numerical data was compared using the Mann-Whitney U test, and categorical data was compared using the Chi-Square test. EFS was defined as the time from diagnosis to removal from the study due to relapse, death or failure to achieve complete remission, or was censored at the last follow-up. OS was defined as the time from diagnosis to death from any cause, or was censored at the last follow-up. EFS and OS were estimated using the Kaplan-Meier method and the log-rank test. Multivariate proportional hazards models were constructed for EFS and OS, using a limited backward elimination procedure. P<0.05 was considered statistically significant for all analyses. All statistical tests were two sided and performed by SPSS software 20.0 and GraphPad Prism software 7.0.
Results
Association of different expression levels of the PANK members with EFS and OS in AML patients
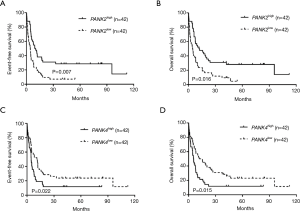
To evaluate the association of the PANK family expression and survival of the AML patients who only received chemotherapy, Kaplan-Meier method and the log-rank test were performed and the results were presented in Table 1. High expression levels of PANK2 and PANK4, i.e., the expression levels were higher than the median in the group, had associations with survival. Specifically, patients with high PANK2 expression had longer EFS and OS than those with low PANK2 expression (P=0.007, P=0.016, respectively, Figure 1A,B); patients with high PANK4 expression had shorter EFS and OS than those with low PANK4 expression (P=0.022, P=0.015, respectively, Figure 1C,D).

Full table
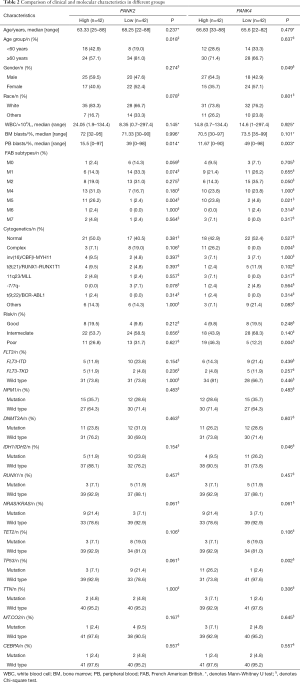
Comparison of the clinical and molecular characteristics in different expression groups of PANK2 and PANK4
According to the median expression levels of PANK2 or PANK4, all patients were divided into high and low expression groups respectively. Comparison of the clinical and molecular characteristics between different groups were summarized in Table 2. The PANK2high group had more patients with age <60 years (P=0.018), lower PB blasts percentage (P=0.014), more FAB-M5 patients (P=0.004), and fewer patients with complex karyotype than the PANK2low group. No significant differences were found in gender distribution, race, peripheral WBC count, BM blasts, risk-group distribution and frequencies of common genetic mutations (FLT3-ITD, NPM1, DNMT3A, RUNX1, TET2, TP53, TTN, MT-CO2, CEBPA, IDH1/IDH2, NRAS/KRAS) between the two groups. The PANK4high group had more male patients (P=0.049), lower PB blasts percentage (P=0.003), more patients with complex karyotype (P=0.004), more patients with poor-risk cytogenetics (P=0.004), lower mutation frequency of IDH1/IDH2 (P=0.046) and higher frequency of TP53 (P=0.002). No significant differences were found in age, race, peripheral WBC count, BM blasts, FAB subtypes and frequencies of other common genetic mutations (FLT3-ITD, NPM1, DNMT3A, RUNX1, TET2, TTN, MT.CO2, CEBPA, NRAS/KRAS) between the two groups.
Full table
Prognostic value of PANK2 and PANK4 expression in AML patients
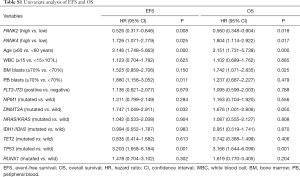
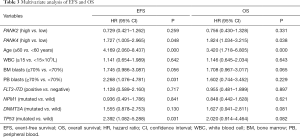
To further evaluate prognostic value of PANK2 and PANK4, multivariate Cox proportional hazard models were constructed, involving factors that had significant association (P<0.05) with EFS and/or OS in the Univariate analysis (Table S1), such as the expression levels of PANK2 and PANK4 (high vs. low), age (≥60 vs. <60 years), BM blast percentage (≥70% vs. <70%), PB blast percentage (≥70% vs. <70%) and recurrent genetic mutations (DNMT3A and TP53, mutated vs. wild type). We also involved factors that had established survival impact on AML, including peripheral WBC count (≥15×109/L vs. <15×109/L), FLT3-ITD (positive vs. negative), and genetic mutations (NPM1 mutated vs. wild type). Results of the multivariate analysis were shown in Table 3. High PANK4 expression and age ≥60 years were independent risk factors for EFS and OS (all P<0.05). In addition, PB blasts ≥70% and TP53 mutation were independent risk factors for EFS (all P=0.031).
Full table
Full table
Discussion
In this study, we found that high PANK2 expression could have a favorable prognostic impact on AML, although the effect was not independent, but high PANK4 expression definitely played an independently negative role in the survival of AML patients who only received chemotherapy.
Several studies demonstrated a significant role of the PANK family in cancer. A study found that PANK2 can be a therapeutic target for thyroid cancer (11). MiR-103, which is encoded by the PANK3 gene, and is expressed as a biproduct of PANK3, plays an important role in prognosis of leukemia (12). PANK1, a direct transcriptional target of p53, is associated with the regulation of energy homeostasis in human colon cancer cells (13). Taken together, these results, although limited, indicated that PANK members may exert important effects on tumorigenesis.
In this study, we found that the PANK2high group had more young patients, lower PB blast percentage and fewer patients with FAB-M5 subtype, suggesting that these factors may have some combined favorable effect in AML. In addition, we found that high PANK4 expression was more common in patients with poor-risk and complex karyotype. This concurred with previous studies that the prognosis of AML patients with complex karyotype and poor-risk is generally poor (15,16). High PANK4 expression was more likely to co-exist with TP53 mutation and FAB-M5 subtype. It is known that FAB-M5 patients usually are more refractory to treatment and have poor prognosis (17), and TP53 mutation are related to adverse prognosis in complex karyotype AML (18). Moreover, high PANK4, but not high PANK2 expression, was an independent risk factor for EFS and OS, demonstrated by multivariate analysis. Hence, high expressions of PANK2 and PANK4 all can affect the prognosis of AML patients, but high PANK4 expression has a stronger effect.
The mechanisms of the tumorigenic role of PANK2 and PANK4 are under investigation. Zhou et al. reported that abnormal expression of PANK2 leads to aggregation of cysteine and synthesis of oxygen free radicals, further regulating oxidative stress inside cells (19). AML cells are susceptible to oxidative stress due to low spare reserve capacity in their respiratory chain (20), therefore PANK2 may affect the survival of patients by enhancing the oxidative stress in leukemic cells and promote cell death. Previous research also reported that overexpression of PANK4 could inhibit cell apoptosis in the pancreas by decreasing the transcriptional level of pro-caspase-9 (21). Combined with our results, we speculated that high PANK4 expression might also suppress AML cell apoptosis by inhibiting pro-caspase-9 expression, leading to shorter survival of patients.
In addition to the above findings, in multivariate analysis, we also found that age ≥60 years, PB blasts ≥70% and TP53 mutation had adverse effects on EFS and OS, consistent with the facts that elderly AML patients usually had adverse prognosis due to higher mutation frequency, poorer baseline performance status and more comorbidities (22), and the well-established knowledge that TP53 mutation conferred adverse prognosis in complex karyotype AML (16). In studies of childhood acute lymphoblastic leukemia, PB blast percentage is an adverse prognostic factor, yet this role has not been well-recognized in AML (23).
Conclusions
In summary, our study revealed that high expressions of PANK2 was associated with better survival, but PANK4 was an independent poor prognostic factor, in AML patients who only received chemotherapy. Due to the limitation of sample size, our study would require further verification by large prospective cohorts.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (81500118, 61501519, 81800983), the China Postdoctoral Science Foundation funded project (Project No.2016M600443), Jiangsu Province Postdoctoral Science Foundation funded project (Project No. 1701184B), PLAGH project of Medical Big Data (Project No. 2016MBD-025).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consents were obtained from all patients, and the study protocol was approved by the Human Research Council of University of Washington.
References
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell 2014;14:275-91. [Crossref] [PubMed]
- Cazzola M, Della Porta MG, Travaglino E, et al. Classification and prognostic evaluation of myelodysplastic syndromes. Semin Oncol 2011;38:627-34. [Crossref] [PubMed]
- Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008;111:2776-84. [Crossref] [PubMed]
- Terada K, Yamaguchi H, Ueki T, et al. Full-length mutation search of the TP53 gene in acute myeloid leukemia has increased significance as a prognostic factor. Ann Hematol 2018;97:51-61. [Crossref] [PubMed]
- Ho PA, Alonzo TA, Gerbing RB, et al. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children's Oncology Group. Blood 2009;113:6558-66. [Crossref] [PubMed]
- Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005;106:3733-9. [Crossref] [PubMed]
- Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012;481:506-10. [Crossref] [PubMed]
- Mrozek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood 2007;109:431-48. [Crossref] [PubMed]
- Bjorkelid C, Bergfors T, Raichurkar AK, et al. Structural and biochemical characterization of compounds inhibiting Mycobacterium tuberculosis pantothenate kinase. J Biol Chem 2013;288:18260-70. [Crossref] [PubMed]
- Leonardi R, Zhang YM, Rock CO, et al. Coenzyme A: back in action. Prog Lipid Res 2005;44:125-53. [Crossref] [PubMed]
- Iacobas DA, Tuli NY, Iacobas S, et al. Gene master regulators of papillary and anaplastic thyroid cancers. Oncotarget 2017;9:2410-24. [Crossref] [PubMed]
- Kfir-Erenfeld S, Haggiag N, Biton M, et al. miR-103 inhibits proliferation and sensitizes hemopoietic tumor cells for glucocorticoid-induced apoptosis. Oncotarget 2017;8:472-89. [Crossref] [PubMed]
- Wang SJ, Yu G, Jiang L, et al. p53-Dependent regulation of metabolic function through transcriptional activation of pantothenate kinase-1 gene. Cell Cycle 2013;12:753-61. [Crossref] [PubMed]
- Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059-74. [Crossref] [PubMed]
- Perrot A, Luquet I, Pigneux A, et al. Dismal prognostic value of monosomal karyotype in elderly patients with acute myeloid leukemia: a GOELAMS study of 186 patients with unfavorable cytogenetic abnormalities. Blood 2011;118:679-85. [Crossref] [PubMed]
- Bowen D, Groves MJ, Burnett AK, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 2009;23:203-6. [Crossref] [PubMed]
- Darmon M, Azoulay E. Prognosis of acute monocytic leukemia (French-American-British classification M5). J Clin Oncol 2005;23:1327-author reply 1327-8. [Crossref] [PubMed]
- Rucker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012;119:2114-21. [Crossref] [PubMed]
- Zhou B, Westaway SK, Levinson B, et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet 2001;28:345-9. [Crossref] [PubMed]
- Sriskanthadevan S, Jeyaraju DV, Chung TE, et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood 2015;125:2120-30. [Crossref] [PubMed]
- Xiang RL, Yang YL, Zuo J, et al. PanK4 inhibits pancreatic beta-cell apoptosis by decreasing the transcriptional level of pro-caspase-9. Cell Res 2007;17:966-8. [Crossref] [PubMed]
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med 2015;373:1136-52. [Crossref] [PubMed]
- de Jonge HJ, Valk PJ, de Bont ES, et al. Prognostic impact of white blood cell count in intermediate risk acute myeloid leukemia: relevance of mutated NPM1 and FLT3-ITD. Haematologica 2011;96:1310-7. [Crossref] [PubMed]


