
Treatment and clinical outcomes of cervical cancer during pregnancy
Introduction
Cervical cancer is the most common gynecological malignant cancer diagnosed during pregnancy (1). According to the patient population studied, cervical cancer during pregnancy (CCP) was reported in approximately every 10,000 pregnant women (2). Among all cervical cancer patients, approximately 1–3% of patients were pregnant at the time of diagnosis (1,3). As the management of CCP is complex, a multidisciplinary discussion is imperative among gynecological surgeons, medical oncologists, radiation oncologists, pathologists, obstetricians, neonatologists, and patients themselves (4).
For CCP treatment, physicians have to consider both fetal preservation (if possible) and the potential loss of reproductive ability as a consequence of cancer therapy. Due to the difficulties encountered in the treatment of CCP, there is currently no unified treatment guide around the world. Additionally, there is much controversy in the handling of CCP. Amant et al. recommended that the management of CCP should refer to French and European consensus meeting guidelines (1). Additionally, optimal oncologic therapy, as well as preservation of healthy fetuses, should also be taken into consideration. Treatment options including conservative and surgical approaches are based on tumor size; lymph node involvement, gestational age (GA), and the patients’ wish to continue the pregnancy or not (5). Still, many questions about the prognosis and management of CCP remain unanswered. Does pregnancy have a deleterious effect on the prognosis of cervical cancers? Under what circumstances can the treatments be delayed until fetal maturity? This being the case, a retrospective review of 92 CCP patients was performed to help solve these questions.
Methods
Patients
A total of 92 patients with CCP were enrolled in this study. One patient diagnosed in 2006 was identified by the Gynecology Department of Nanfang Hospital of Southern Medical University (Guangzhou, Guangdong Province, China). Five patients diagnosed from 2001 to 2006 were admitted to the Gynecology Department of Tongji Hospital Affiliated to Huazhong University of Science and Technology (Wuhan, Hubei Province, China). The remaining 86 patients were collected from case-series reported in the literature from the PubMed database from January 1961 to January 2019 (6-11), using the search terms “pregnancy” and “cervical cancer.” All patients were selected based on the following criteria: availability of conclusive histopathologic diagnosis as adenocarcinoma (AC), squamous and rare subtypes including small-cell carcinoma (12-14), large-cell neuroendocrine cervical carcinoma (15), rhabdomyosarcoma (16), clear cell carcinoma (17), and glassy cell cervical carcinoma (18); no previous malignant disease or a second primary tumor; and with complete clinical pathology and follow-up data. The diagnosis of cervical cancer was confirmed using a biopsy.
By reviewing the individual patient demographic and tumor characteristics, an attempt was made to exclude cases that may have been included in two or more publications. The individual patient data were abstracted from the text and tables in the publications but not extrapolated from the figures. Patients and disease characteristics were evaluated, including the age of diagnosis, 2018 International Federation of Gynecology and Obstetrics (2018 FIGO) stage, lymph node involvement, lymph-vascular space invasion, tumor size, histological subtype, treatment strategies, mode of delivery, and neonatal outcomes. This study was approved by the Medical Ethics Committee of Nanfang Hospital of Southern Medical University. Informed consents were obtained from the parents of the six patients enrolled.
Statistical analysis
Statistical analysis was performed using SPSS 22.0. Kaplan-Meier life table analyses were used to analyze the significant clinical and pathologic risk factors for survival. Independent prognostic factors predictive of survival were analyzed using Cox regression methods. All tests were two-tailed, and P value <0.05 was considered significant.
Results
Basic clinical information of 92 CCP patients
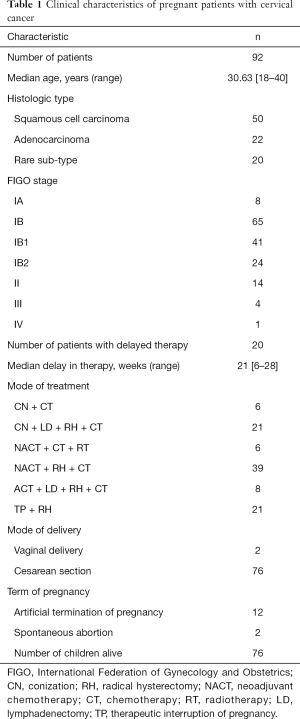
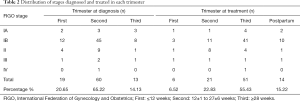
To synthetically investigate prognostic factors of survival for the enrolled patients, demographic and tumor characteristics were assessed (Table 1). The predominant histological cell type for the patients was squamous cell carcinoma (SCC) (19). Most patients were grouped into 2018 FIGO stage IB (41 IB1 and 24 IB2) (20). The distribution of stages diagnosed and treated in each trimester was then studied (Table 2). Most diagnoses were made during the second trimester (60 patients, 65.22%) and most treatments were performed in the third trimester (41 patients, 44.57%). For 24 patients, the treatments were performed during the second or third trimester to prolong GA. The average GA week of these 24 patients was 35±2 weeks. Thirty-six patients underwent neoadjuvant chemotherapy (NACT) (8-11,21-25).
Full table
Full table
Survival outcomes
Among the 92 CCP patients, except for 12 patients (reported cases) (26,27) whose pregnancies were terminated by artificial termination, two of the patients suffered from spontaneous abortion (6,28), one baby experienced acute myeloid leukemia (AML) ex-FAB (French-American-British)-M7 subtype and received bone marrow transplantation, and two cases were not mentioned in articles (3,29). The other 75 had viable children without abnormality or malformation (Table 1). The most frequent mode of delivery (76 patients, 82.6%) was a cesarean section. Two patients had a vaginal delivery with neonatal death (6,30). The range of gestation was 26±2 to 38±3 weeks.
Of the 12 patients who selected artificial termination of pregnancy after CCP was confirmed by pathological biopsy, one was a rare pathological type, two cases were AC, and the rest were SCC. For the nine cases of SCC, one case was in 2018 FIGO stage IIIA stage; therefore, radical surgery was selected. Seven patients were in 2018 FIGO stage IB2 (tumor diameter >2 cm) and one patient was in 2018 FIGO IB1. Radical hysterectomies with cesarean sections were performed in these 12 cases.
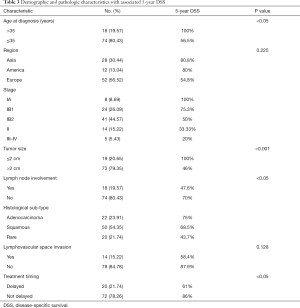
To illustrate whether pregnancy has a deleterious effect on the prognosis of cervical cancers, we divided 92 patients into two groups according to treatment timing (Table 3): one underwent treatment delay until fetal maturity (delayed treatment); the other received treatment immediately when diagnosed of CCP (not delayed) (31), including treatment after pregnancy termination or during pregnancy (32). The 5-year DSS for delayed treatment was 61%. Meanwhile, the no delay group was 86% (P<0.05). There was a significant difference in survival whether the treatment was performed once diagnosed or not.
Full table
Other risk factors for patient survival
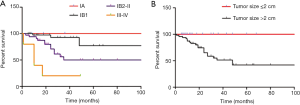
To analyze the relationship between 5-year DSS and tumor characteristics, the follow-up survey was studied (Table 3). The 5-year DSS of patients with tumor size ≤2 and >2 cm was quite different (P<0.001; Figure 1). In multivariate analysis, tumor size was an independent prognostic factor for improved survival (P<0.05; Table 4). In addition, as to patients with information on lymph node involvement, the 5-year DSS for lymph node or no lymph node dissection was 47.6% and 70% respectively (P<0.05). For histological subtype, the 5-year DSS for SCC, AC, and rare sub-type was 68.5%, 75%, and 43.7% respectively.

Full table
Treatment modalities
To look for better strategies, treatment programs of the 92 cases were evaluated. Radical hysterectomy with pelvic lymphadenectomy, which is widely used in total hysterectomy and lymph node dissection and is the most important surgical procedure, was performed immediately after cesarean section. In this study, 80 patients (86.96%) underwent the procedure, among whom 39 received neoadjuvant chemotherapy before the operation. From the literature collected, 19 patients (20.65%) were found to have lymphatic metastasis (30,33,34). Eight patients received 2–4 cycles of adjuvant chemotherapy either combined with lymphadenectomy during the second and third trimester. NACT was the primary treatment in patients with stage IB and III–IV for 54 patients. Among the 54 patients, one patient suffered from spontaneous abortion, and one baby experienced AML ex-FAB (French-American-British)-M7 subtype and received bone marrow transplantation. The other 51 fetuses were safely delivered by cesarean section (Table 1).
Discussion
Emerging evidence has indicated that cervical cancer is the most frequently diagnosed malignancy during pregnancy (1). Cervical cancer during pregnancy represents an important challenge because of its impact on fetal development, difficulties in CCP management, and unknown oncologic outcomes (35). Thus, we sought to investigate clinicopathologic factors associated with survival rate and the management of CCP so as to provide alternatives for CCP treatment. From 92 patients enrolled in this report, we arrived at three conclusions that were different from those previously reported. First, our data showed that tumor size was an independent prognostic factor. Second, pregnancy may be harmful to the progression of cervical cancer. Finally, our report showed little benefit associated with chemotherapy in the treatment of this disease.
The key prognostic predictors of CCP patients are GA, local extension, histological subtype, and lymph node involvement (4,36,37). Previous analyses identified the stage of this disease as the only significant prognostic factor (38-40). However, in this report, the majority of patients were stage I (79.35%); therefore, the stage showed little relevance with patient survival in this case (P=0.201). Tumor size was an independent prognostic factor (P<0.05), suggesting that whether the tumor size >2 or ≤2 cm was significant to the survival of CCP patients. It is well known that tumor size and lymph node involvement are independent prognostic factors for CCP; however, lymph node metastasis was not identified as an independent factor. We considered that this might be caused by the limited samples enrolled in this study.
Regarding histological subtypes, Morice et al. (24) argued that conventional subtypes such as squamous-cell, AC, and adenosquamous lesions have the same prognosis (37). Rare subtypes such as small-cell carcinoma showed a poor prognosis. Our results showed little difference between squamous and AC subtypes, and the survival of rare subtype cervical cancers is worse than that of cervical SCC and AC in stage III–IV.
There is some controversy in the effects of pregnancy on the progression and prognosis of cervical cancer. Hecking et al. (39) showed that pregnancy could accelerate tumor cell proliferation. The mechanical action of cervical dilatation during delivery made it easy to cause the spread of cancer emboli which are then prone to metastasize through pelvic lymph nodes and blood circulation (41). In contrast to this, however, Morice et al. (37) showed that pregnancy did not affect the survival of women with cervical cancer. Meanwhile, European recommendations state that pregnancy should be preserved whenever feasible (37). However, in our results, the 5-year DSS for patients whose treatment was prolonged to fetal maturity was lower than for those who received treatment immediately after diagnosis. Thus, we suppose that pregnancy may have a deleterious effect on the prognosis of cervical cancer.
Recently, NACT has been administered to patients with locally advanced cancer to improve outcomes (42,43). According to the International Gynecologic Cancer Society (IGCS) and the European Society of Gynecologic Oncology (ESGO) Guidelines (1), neoadjuvant chemotherapy until fetal maturity is recommended for patients with FIGO stage IB1 tumors (>2 cm) and negative nodes. Song et al. (44) demonstrated that neoadjuvant platinum-based chemotherapy could be a favorable choice for the management of patients with cervical cancer during the second and third trimesters. To reduce the side effects of chemotherapy, cisplatin might be good to use as monotherapy in these patients. In our study, although adjuvant chemotherapy was the main treatment during the third trimester of pregnancy, 5.26% of the women (2/38) underwent a spontaneous abortion, and one baby experienced AML ex-FAB (French-American-British)-M7 subtype and received bone marrow transplantation. Therefore, one hypothesis is that NACT showed toxicity to the infants. It is difficult to draw the conclusion that adjuvant chemotherapy may improve the overall survival and progression-free survival of CCP patients. However, NACT followed by RH for the treatment of locally invasive cervical cancer has emerged as an alternative to concurrent chemo-radiotherapy (22,25) in recent years. It is also currently the standard therapeutic approach (3). Future studies aimed at characterizing the effectiveness and deleteriousness for mothers may be warranted.
For CCP management, radical vaginal trachelectomy is commonly considered as a potential treatment option in pregnant women with a desire to continue the pregnancy (45,46). Regarding radical trachelectomy [abdominal (7,47) or vaginal (45)] combined lymphadenectomy, only 12 cases were described during pregnancy. Analogously, previous guidelines of a second international consensus meeting (48) claimed it as a technically challenging procedure, which is associated with massive blood loss and prolonged surgery. Thus, based on our data, we do not suggest radical trachelectomy during pregnancy. Hence, a prospective study is needed to verify whether radical hysterectomy would be proper for fetuses and mothers.
In conclusion, tumor size was an independent prognostic factor of survival in CCP patients. Treatment after delivery could be proposed to selected CCP patients with tumor size ≤2 cm and negative lymph nodes. Once tumor size >4 cm was identified, NACT should be performed as soon as possible, and RH should be taken after childbirth. In summary, pregnancy did have a deleterious effect on the prognosis of cervical cancers. Physicians may design therapeutic approaches according to the patient’s desire, tumor size, GA, and lymph node involvement. Given these conclusions, personalized treatment is strongly recommended.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China [grant numbers: 81672589, 81372871, 81072132], the Shenzhen Science and Technology Program [grant number: JCYJ20160429161218745], and the National Key Research and Development Program of China [grant numbers: 2016YFC1302900].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Medical Ethics Committee of Nanfang Hospital of Southern Medical University. Informed consents were obtained from the parents of the six patients enrolled.
References
- Amant F, Halaska MJ, Fumagalli M, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer 2014;24:394-403. [Crossref] [PubMed]
- Can NT, Robertson P, Zaloudek CJ, et al. Cervical squamous cell carcinoma metastatic to placenta. Int J Gynecol Pathol 2013;32:516-9. [Crossref] [PubMed]
- Fruscio R, Villa A, Chiari S, et al. Delivery delay with neoadjuvant chemotherapy for cervical cancer patients during pregnancy: a series of nine cases and literature review. Gynecol Oncol 2012;126:192-7. [Crossref] [PubMed]
- Germann N, Haie-Meder C, Morice P, et al. Management and clinical outcomes of pregnant patients with invasive cervical cancer. Ann Oncol 2005;16:397-402. [Crossref] [PubMed]
- Van De Nieuwenhof HP, van Ham MA, Lotgering FK, et al. First case of vaginal radical trachelectomy in a pregnant patient. Int J Gynecol Cancer 2008;18:1381-5. [Crossref] [PubMed]
- Favero G, Chiantera V, Oleszczuk A, et al. Invasive cervical cancer during pregnancy: laparoscopic nodal evaluation before oncologic treatment delay. Gynecol Oncol 2010;118:123-7. [Crossref] [PubMed]
- Hecking T. Individual management of cervical cancer in pregnancy. 2016;293:931-9.
- Dipple A, Pigott MA. Resistance of 7,12-dimethylbenz[a]anthracene-deoxyadenosine adducts in DNA to hydrolysis by snake venom phosphodiesterase. Carcinogenesis 1987;8:491-3. [Crossref] [PubMed]
- De Vincenzo R, Tortorella L, Ricci C, et al. Locally advanced cervical cancer complicating pregnancy: A case of competing risks from the Catholic University of the Sacred Heart in Rome. Gynecol Oncol 2018;150:398-405. [Crossref] [PubMed]
- Kayahashi K, Mizumoto Y. A successful case of neoadjuvant chemotherapy and radical hysterectomy during pregnancy for advanced uterine cervical cancer accompanied by neonatal erythroderma. J Obstet Gynaecol Res 2018;44:2003-7. [PubMed]
- Marchiolè P, Ferraioli D, Moran E, et al. NACT and laparoscopic-assisted radical vaginal trachelectomy in young patients with large (2-5cm) high risk cervical cancers: Safety and obstetrical outcome. Surg Oncol 2018;27:236-44. [Crossref] [PubMed]
- Leung TW. Small cell carcinoma of the cervix complicated by pregnancy. Clin Oncol (R Coll Radiol) 1999;11:123-5. [PubMed]
- Perrin L. Small cell carcinoma of the cervix of neuroendocrine origin causing obstructed labour. Aust N Z J Obstet Gynaecol 1996;36:85-7. [PubMed]
- Liu H. Small cell carcinoma of the cervix at 32-week gestation: a case report and review of the literature. Lab Med 2014;45:52-5. [PubMed]
- Li WW. Large-cell neuroendocrine carcinoma of the uterine cervix complicating pregnancy. Hong Kong Med J 2009;15:69-72. [PubMed]
- Meseci E. Embryonal rhabdomyosarcoma of the uterine cervix in a pregnant woman. Taiwan J Obstet Gynecol 2014;53:423-5. [PubMed]
- Terada T. Clear cell adenocarcinoma of the uterine cervix in a young pregnant woman: a case report with immunohistochemical study. Med Oncol 2011;28:290-3. [PubMed]
- Balderston KD, Tewari K, Gregory WT, et al. Neuroendocrine small cell uterine cervix cancer in pregnancy: long-term survival following combined therapy. Gynecol Oncol 1998;71:128-32. [Crossref] [PubMed]
- Sioutas A. Three cases of vaginal radical trachelectomy during pregnancy. Gynecol Oncol 2011;121:420-1. [PubMed]
- Lai CH. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol 1997;64:456-62. [PubMed]
- Caluwaerts S. Neoadjuvant chemotherapy followed by radical hysterectomy for invasive cervical cancer diagnosed during pregnancy: report of a case and review of the literature. Int J Gynecol Cancer 2006;16:905-8. [Crossref] [PubMed]
- Tewari K. Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: a report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer 1998;82:1529-34. [PubMed]
- Benhaim Y. Neoadjuvant chemotherapy for advanced stage cervical cancer in a pregnant patient: report of one case with rapid tumor progression. Eur J Obstet Gynecol Reprod Biol 2008;136:267-8. [PubMed]
- Palaia I. Neoadjuvant chemotherapy plus radical surgery in locally advanced cervical cancer during pregnancy: a case report. Am J Obstet Gynecol 2007;197:e5-6. [PubMed]
- Karam A. Neoadjuvant cisplatin and radical cesarean hysterectomy for cervical cancer in pregnancy. Nat Clin Pract Oncol 2007;4:375-80. [PubMed]
- Clark JF. Invasive carcinoma of the cervix coincident with pregnancy: report of a case. J Natl Med Assoc 1960;52:169-70. [PubMed]
- Taghavi K. Wrong place at the wrong time: A case of cervical embryonal rhabdomyosarcoma in pregnancy. Gynecol Oncol Rep 2015;12:77-9. [PubMed]
- Sopracordevole F, Rossi D, Di Giuseppe J, et al. Conservative Treatment of Stage IA1 Adenocarcinoma of the Uterine Cervix during Pregnancy: Case Report and Review of the Literature. Case Rep Obstet Gynecol 2014;2014:296253. [Crossref] [PubMed]
- Peculis LD. Stage IB2 adenosquamous cervical cancer diagnosed at 19-weeks' gestation. Aust N Z J Obstet Gynaecol 2015;55:94-7. [PubMed]
- Boyd A. The use of cisplatin to treat advanced-stage cervical cancer during pregnancy allows fetal development and prevents cancer progression: report of a case and review of the literature. Int J Gynecol Cancer 2009;19:273-6. [PubMed]
- Kobayashi Y. A case of successful pregnancy after treatment of invasive cervical cancer with systemic chemotherapy and conization. Gynecol Oncol 2006;100:213-5. [PubMed]
- Bader AA. Long-term follow-up after neoadjuvant chemotherapy for high-risk cervical cancer during pregnancy. Gynecol Oncol 2007;105:269-72. [PubMed]
- Marana HR. Chemotherapy in the treatment of locally advanced cervical cancer and pregnancy. Gynecol Oncol 2001;80:272-4. [Crossref] [PubMed]
- Canto MJ. Metastatic small-cell carcinoma of the cervix during pregnancy. J Obstet Gynaecol 2014;34:204-5. [PubMed]
- Lin CH. Successful conservative treatment of microinvasive cervical cancer during pregnancy. J Chin Med Assoc 2013;76:232-4. [PubMed]
- Abu-Rustum NR, Tal MN, DeLair D, et al. Radical abdominal trachelectomy for stage IB1 cervical cancer at 15-week gestation. Gynecol Oncol 2010;116:151-2. [Crossref] [PubMed]
- Morice P, Uzan C, Gouy S, et al. Gynaecological cancers in pregnancy. Lancet 2012;379:558-69. [Crossref] [PubMed]
- Ferriaoli D, Buenerd A, Marchiole P, et al. Early invasive cervical cancer during pregnancy: different therapeutic options to preserve fertility. Int J Gynecol Cancer 2013;23:586. [Crossref] [PubMed]
- Hecking T, Abramian A, Domrose C, et al. Individual management of cervical cancer in pregnancy. Arch Gynecol Obstet 2016;293:931-9. [Crossref] [PubMed]
- Lai CH, Hsueh S, Chang TC, et al. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol 1997;64:456-62. [Crossref] [PubMed]
- Ribeiro F. Cervical cancer in pregnancy: 3 cases, 3 different approaches. J Low Genit Tract Dis 2013;17:66-70. [PubMed]
- Giacalone PL. Cis-platinum neoadjuvant chemotherapy in a pregnant woman with invasive carcinoma of the uterine cervix. Br J Obstet Gynaecol 1996;103:932-4. [PubMed]
- Li J. Neoadjuvant chemotherapy with paclitaxel plus platinum for invasive cervical cancer in pregnancy: two case report and literature review. Arch Gynecol Obstet 2011;284:779-83. [PubMed]
- Song Y, Liu Y, Lin M, et al. Efficacy of neoadjuvant platinum-based chemotherapy during the second and third trimester of pregnancy in women with cervical cancer: an updated systematic review and meta-analysis. Drug Des Devel Ther 2018;13:79-102. [Crossref] [PubMed]
- van de Nieuwenhof HP. First case of vaginal radical trachelectomy in a pregnant patient. Int J Gynecol Cancer 2008;18:1381-5. [PubMed]
- Mandic A. Radical abdominal trachelectomy in the 19th gestation week in patients with early invasive cervical carcinoma: case study and overview of literature. Am J Obstet Gynecol 2009;201:e6-8. [PubMed]
- Abu-Rustum NR. Radical abdominal trachelectomy for stage IB1 cervical cancer at 15-week gestation. Gynecol Oncol 2010;116:151-2. [PubMed]
- Mandic A, Novakovic P, Nincic D, et al. Radical abdominal trachelectomy in the 19th gestation week in patients with early invasive cervical carcinoma: case study and overview of literature. Am J Obstet Gynecol 2009;201:e6-8. [Crossref] [PubMed]


