
Successful therapy for autoimmune myocarditis with pembrolizumab treatment for nasopharyngeal carcinoma
Introduction
Immune checkpoint inhibitors (ICIs) has been widely accepted by oncologists for its significant treatment effects for numerous cancers such as melanoma, non-small cell lung cancer, head and neck cancer and tumors of any organ with high microsatellite instability, as it improves the survival in a percentage of patients with numerous tumors (1). These effective drugs also bring about wide spectrum of adverse events, including rashes, colitis and hepatitis (2). The incidence of pneumonitis and myocarditis cause by ICI’s were related lower compared with other immune-related adverse events (irAEs), these toxic effects are considerably more severe and fulminant (3,4), and also have high case fatality rate (3). In this report, we describe a case of myocarditis accompanied with pneumonia after the treatment of pembrolizumab for metastatic nasopharyngeal carcinoma.
Case presentation
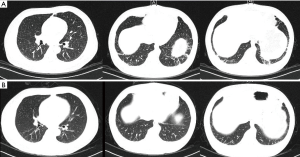
A 45-year-old man with advanced nasopharyngeal cancer presented in the emergency department on August 7th 2018 after having fever, with temperatures reaching 38.9 °C, left knee pain, rashes on his hips, muscles pain and fatigue for 2 days, which means 4 days after the first stage of pembrolizumab treatment (1.3 mg per kilogram of body weight). He had no remission of symptoms after being treated with moxifloxacin and cefminox for 1 day and suffered from rashes on his trunk when he was injected with moxifloxacin which was regarded as moxifloxacin allergy. Then he was admitted to our infectious diseases department on admission, he had a fever of 39.2 °C, the heart rate was up to 110 bpm, and blood pressure and breath rate were within the normal range. The physical examination revealed several enlarged lymph nodes on the right submandibular area, the diameter of the largest one was almost 2 centimeters, which had an oval shape and a rubbery quality. A well-defined, raised erythema and crusting were presented on the hips. The laboratory results on admission were peripheral-blood leukocyte count of 9,400 per cubic millimeter with 84.4% neutrophils, erythrocyte sedimentation rate of 45 mm per h (mm/h, normal range, 0–20 mm per h), high-sensitivity C-reactive protein (hs-CRP) of 194.2 milligram per litre (mL/L),procalcitonin of 0.58 nanogram per milliliter (ng/mL), troponin T level (cTNT) and N-terminal pro-brain natriuretic peptide (NT-proBNP) were elevated to 0.038 ng/mL (normal level <0.03) and 1,160 picograms per milliliter (pg/mL, normal level 0–100) respectively. Within 24 h, the levels of cTNT and NT-proBNP were elevated to 0.045 ng/mL and 1,160 pg/mL, respectively. Creatine kinase (CK) and creatine kinase-myocardial band (CK-MB) were normal. There were mild elevation of liver enzymes with alanine transaminase (ALT) level of 56 (normal range, 9–50) U/L. Viral antibody titers were negative except Epstein-Barr virus-related IgA (EBV-IgA), EBV-IgM (which was positive before immunotherapy) and coxsackieviruses B-IgM and IgG, the antinuclear antibody titer was 1:100; Blood cultures were all negative twice and thyroid stimulating hormone levels were negative. The arterial partial pressure of oxygen breathing ambient air was 67 mmHg. The electrocardiogram (ECG) showed the ST segment was elevated by 2–3 mm in V1–V3 and deep T wave inversion in I, aVL, V4, V5, V6, which revealed notable changes compared with the ECG before admission (Figure 1). The echocardiogram (Echo) revealed the increased thickness of the left ventricular wall (LVW) and left ventricular posterior wall (LVPW), although there was no decrease in systolic left ventricular (LV) and right ventricular (RV) function. Given the patient’s hypoxia, we performed a chest CT, which showed increasing patchy ground opacities and fibrous stripes of the bilateral lower lobe (chest CT changes shown in Figure 2).
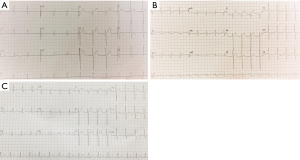
Given the elevated biomarkers for cardiac injury and ECG, Echo changes, we immediately received a cardiology consultation, which suggested that the slight changes of ST segments may be related to the leads locations and the mild increase of cTNT may be correlated with severe infection. To prevent the progression of disease, we prescribe antibiotics and antivirus drug on D1. Meanwhile, the patient still suffered from fever without any release, and the levels of CRP and procalcitonin (PCT) were rising. The coronary computed tomography angiography showed no obstructive disease, and the peripheral-blood was tested by metagenomic next generation sequencing (the negative result was reported on D4). Endomyocardial biopsy (EMB) was not performed due to the strong disagreement of the patient and his family.
Given the high temperature, increased level of biomarkers, myocardial injury manifested by changes of cardiac biomarkers, ECG and Echo, pneumonitis revealed by chest CT, the lack of clinical improvement following and the correlation of pembrolizumab treatment, autoimmune-induced myocarditis and pneumonitis were the most likely diagnosis.
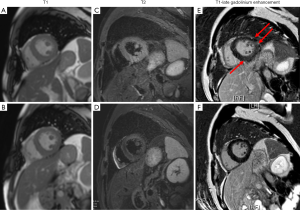
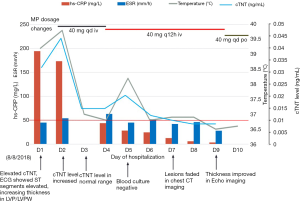
Methylprednisolone (MP) 20 mg was prescribed on D2, and the temperature went back to the normal range. The level of cTNT went back to normal on D3 and the values of NT-proBNP, CRP, erythrocyte sedimentation rate (ESR) and PCT trended downwards over the next several days. Upon consultation with an oncologist, we added a dose of methylprednisolone up to 40 mg qd. On D5, the patient had a fever, with temperature up to 38.2 °C, and the methylprednisolone dosage was increased to 40 mg q12h (shown in Figure 3). On D6, the chest CT showed a reduction of these lesions compared with that of CT on D1. On D7, the erythema faded and the PaO2 increased to 83 mmHg breathing ambient air. On D9, cardiac magnetic resonance (CMR) imaging revealed uneven local thickening of LVW and LVPW and patched myocardial signal intensity increase in T2-weighted fat suppression (FS) images and a patchy lesion in LV inferior wall and ventricular septum in delayed contrast-enhanced T1 imaging indicating myocardial inflammation. On D10, he discharged with the Methylprednisolone dosage to 40 mg qd po. Seven days after discharge, he returned to the hospital for a check and underwent CMR which showed myocardial injury remission (the images compared to the prior CMR are shown in Figure 4).
Discussion
We report an irAEs case in which the mainly symptom was fever. The key to diagnosing myocarditis and pneumonitis caused by ICIs is to exclude other etiologies such as infectious diseases, which immunosuppressive patients are prone to. The onset of irAEs can be as soon as few days and as long as years after initiation of therapy. The clinical presentation and pathology are non-specific, on which basis, the diagnosis of irAEs is considerably more difficult than that of other tumor complications.
Viral infection is one of the prevalent etiologies of myocarditis. Meanwhile, the clinical presentation of viral myocarditis varies from asymptomatic to electrocardiographic abnormalities to shock. The viral prodrome including fever and respiratory or gastrointestinal symptoms precedes the onset of the disease and the related viral load is much higher, which can be negative in other cases of myocarditis. These features are effective measures to distinguish viral myocarditis and immunotherapy related myocarditis.
Myocarditis is one of the rare complications of immune checkpoint inhibitors and is characterized of fulminant and high mortality. A meta-analysis (5) found that the mortality of cardiovascular toxicities is relatively higher than others, i.e., 16% of patients treated with a single dose of pembrolizumab. The mortality is also considerably higher in patients treated with a combination anti-programmed death-ligand 1 (PD-L1). Overall, the case fatality rate was 35%. Early diagnosis and treatment are crucial. However, there are no data available to predict or monitor the evolution of myocarditis. In our case, we kept supervising symptoms, the level of troponin and biomarkers including CRP, ESR, PCT and ECG. Surprisingly, we detected that the patient’s temperature and cTNT went back to normal range on the first day of MP treatment, which manifested that myocarditis was much more sensitive to MP. Continuous monitoring is still crucial in case of relapse according to our experience. According to the data, myocarditis, heart failure arrhythmia and conduction abnormalities are the common types of cardiotoxicity. And in 51% of the heart failure cases, LVEF is abnormal (6), which may be the preserved EF heart failure. And the common causes of death were refractory ventricular arrhythmia and heart failure (7). While in this report, arrhythmia was not detected which may indicate that it is a relatively gentle cardiac complication, which may represent a possible indicator for prognosis.
According to the current statement of diagnosis of myocarditis from the European Heart Journal (8), EMB is the diagnostic gold standard of definite myocarditis, which is used infrequently due to its high risk. Non-invasive techniques such as CMR, are recommended to make the diagnosis of myocarditis or to supervise myocarditis progression (9). The Lake Louise criteria (10) including markers for hyperemia, edema and inflammatory scarring, have been widely adapted to identify acute myocarditis, and the diagnostic accuracy is close to 80%. Moreover, CMR especially the presence of LGE is associated with the prognosis of acute myocarditis (11), which provides a unique value to predict outcome.
In addition to the myocarditis, pneumonitis is another severe and rare immune-related adverse event. To date, the overall incidence is 2.7–5% (12,13) and the difficulty of diagnosis is the lack of specific clinical or radio imaging characteristics. Excluding infection is the primary goal. According to the symptoms such as fever and hypoxemia, the added patchy shadows and elevated inflammation biomarkers, it is difficult to exclude pneumonia. After the treatment of steroids, the hypoxemia improved and the lesions in the chest CT images faded within only 5 days, which indicates the diagnosis of pneumonitis.
The utilization and duration of steroid are highly variable (14). The optimal duration is still unknown due to the difficulty of balancing treatment effects of irAEs and adverse events, although it may depend on the severity of irAEs. Several studies have focused on evaluating prognosis when steroids and ICIs are used simultaneously. A study (15) showed that there was no significant difference in the survival between patients who accepted steroids and those who did not. Scott et al. (16) and Margolin et al.’s studies (17) both showed that there was significant reduction of overall survival in patients accepting steroid compared with who did not. Checkpoint inhibitors with their long half-life, are able to prolong the effect of the drug during which the steroid is tapered to solve mild to moderate irAEs. More research is needed to determine the effect of the concomitant use of steroids with checkpoint inhibitors on the prognosis of carcinoma.
In this report, we described a case of myocarditis and pneumonitis induced by programmed death-1 (PD-1) inhibitors which are rare irAEs with high mortality. Rapid diagnosis and suppression of immune attack using steroids were key to resolving irAEs. This case provides a close monitoring of the ICI induced myocarditis, which provides a reference concerning how to detect irAEs and adjust steroid dosages, especially for cardiology and infectious clinicians.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and for any accompanying images.
References
- Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J Hematol Oncol 2017;10:174. [Crossref] [PubMed]
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190-209. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Frigeri M, Meyer P, Banfi C, et al. Immune Checkpoint Inhibitor-Associated Myocarditis: A New Challenge for Cardiologists. Can J Cardiol 2018;34:92.e1-92.e3. [Crossref] [PubMed]
- Mir H, Alhussein M, Alrashidi S, et al. Cardiac Complications Associated With Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area. Can J Cardiol 2018;34:1059-68. [Crossref] [PubMed]
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755-64. [Crossref] [PubMed]
- Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017;136:2085-7. [Crossref] [PubMed]
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48, 2648a-2648d.
- Biesbroek PS, Hirsch A, Zweerink A, et al. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. Eur Heart J Cardiovasc Imaging 2018;19:1397-407. [Crossref] [PubMed]
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475-87. [Crossref] [PubMed]
- Aquaro GD, Perfetti M, Camastra G, et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol 2017;70:1977-87. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Williams KJ, Grauer DW, Henry DW, et al. Corticosteroids for the management of immune-related adverse events in patients receiving checkpoint inhibitors. J Oncol Pharm Pract 2019;25:544-50. [Crossref] [PubMed]
- Horvat TZ, Adel NG, Dang TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193-8. [Crossref] [PubMed]
- Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J Thorac Oncol 2018;13:1771-5. [Crossref] [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]


