Cytokines expression levels from tissue, plasma or serum as promising clinical biomarkers in adenocarcinoma of the prostate: a systematic review of recent findings
Introduction
Prostate cancer (PC) is a common cancer (excluding non-melanoma skin cancer) in men in many parts of the world, although incidence and mortality rates vary significantly by population. Incidence rates tend to be highest in more developed countries such as North America, Western and Northern Europe, and Australia (1). Conversely, East, Southeast and South-Central Asian men have the lowest incidence and mortality rates from PC (1-3). In Australia, age-standardised incidence and mortality rates for PC were 167/100,000 and 23.4/100,000 respectively in 2010, making it the fourth leading cause of mortality, with 3,294 deaths in 2011 (4,5). From 2001 to 2010, the age-standard incidence rate of PC in the Northern Territory (NT) was 119.4/100,000. This rate was slightly higher for non-Indigenous males (133.3/100,000 when compared to Indigenous males (43.0/100,000) (6). Mortality figures are currently unavailable for Indigenous males with PC but the non-Indigenous mortality rate was 29.3/100,000 from 2001–2006 (6).
Treatment option for PC depends on a number of tumour and patient factors. Radical prostatectomy (RP) and radiotherapy (RT) are two common treatment modalities utilized for the treatment of localized PC. External beam RT is a non-surgical treatment which focusses megavoltage photon beams on the prostate gland, with the aim of loco-regional control, prolonged disease-free survival (DFS) and overall survival (OS) for PC patients (7). RT is can be administered with androgen deprivation therapy (ADT) for those patients with intermediate and high-risk PC (8,9). ADT can also be used during biochemical recurrence after RP, in the presence of pelvic lymph node metastases, and asymptomatic metastatic disease (10).
In current medical practice, prognostic markers for DFS and OS in PC include the presenting serum prostate-specific antigen (PSA) level, tumour Gleason score (GS) and clinical tumour stage (11). Stratification of patients into low, intermediate and high-risk groups can then be used to help select treatment options (11). However, existing pre-treatment factors cannot be used to predict acute RT-induced toxicity. Therefore, new protein biomarkers may be useful in radiation oncology to improve decision-making, treatment and therapy monitoring for PC patients.
Some pro-inflammatory cytokines are believed to play an important role in RT resistance and lead to tumour progression, invasion, and angiogenesis (12-14). Cytokines are water soluble, low molecular weight proteins that transport signals between cells (15). Following RT, researchers believe that normal tissue damage and gene expression changes at the messenger RNA (mRNA) level leads to increased cytokine production within the irradiated area, which then enters the circulation (16,17). Rubin and colleagues were among the first to describe the role of cytokines in mediating RT-induced toxicity. They reported that levels of interleukin (IL)-1, transforming growth factor (TGF)-β, and tumour necrosis factor (TNF)-α were increased immediately after radiation exposure and that elevated TGF-β levels were associated with increased risk of pulmonary fibrosis (18). Christensen et al. reported that interferon-γ (IFN-γ) and interleukin-6 (IL-6) significantly increased during prostate RT with an associated increase in acute gastrointestinal and genitourinary toxicity (19).
This systematic review aims to update the potential research to address the difference in cytokine expression and their association with clinical outcome and RT-induced toxicity by analysing with diagnostic methods such as immunohistochemistry (IHC), enzyme-linked immunosorbent assay (ELISA) and other diagnostic techniques.
Methods
Literature search strategy
This systematic review was conducted using electronic databases (PubMed, Medline, and Google Scholar). In addition, the reference lists of pertinent articles were examined for additional relevant studies. We carried out a systematic search using keywords such as prostate cancer, cytokine expression, IHC, and ELISA and other techniques. In addition, we also used both subject headings and text-word terms for ‘‘radiotherapy’’, ‘‘clinical outcome’’, ‘‘survival/mortality’’, “RT-induced toxicity”.
Studies review method
Articles were retrieved in June 2018 and imported into an Endnote X7 database (20). Duplicate entries were identified and deleted with Endnote’s duplicate function. The remaining articles were sorted alphabetically and then visually scanned to identify any missed duplicates. The abstracts and titles of these articles were carefully identified by the database search and screened to exclude the irrelevant studies. Three authors (JS, PDI and SSS) reviewed potential papers for inclusion. Relevant articles were selected after reading the abstract to determine whether they completely met the inclusion criteria for the systematic review.
Types of participants and treatment
We reviewed studies reporting on PC patients of any age treated with a commonly utilized form of RT including conformal external beam (EBRT), intensity-modulated RT (IMRT), brachytherapy, or a combination of RT modalities with curative treatment intent. We also included studies related to dose and duration of RT. Studied we excluded studies assessing adjuvant or salvage therapies as a specific objective.
Outcome measure
We have selected studies which measured cytokine expression in the patient’s blood plasma or serum and tissue biopsies and correlation with RT-induced toxicity and clinical outcomes of PC patients. We considered studies only reporting multivariable-adjusted hazard ratios (aHR). We excluded crude or unadjusted outcome measures between patients treated with RT and surgery.
Inclusion criteria
Inclusion criteria were (I) studies investigating the association between cytokine expression and clinical outcomes such as DFS and OS; (II) studies investigating cytokine expression and association with RT-induced toxicity; (III) studies using blood plasma or serum and prostatic tissues for cytokines analysis; (IV) the articles must list the sample size, sampling methods, diagnostic techniques, clinicopathological characteristics and clinical outcomes.
Exclusion criteria
Publications such as editorials, commentaries and review articles were excluded. Studies not subject to peer-review were also excluded. If there were more than one study resulting from the same patient cohort, to prevent data duplication, these were also excluded. Animal studies were also excluded.
Data extraction
Data from eligible studies was extracted with the following information by two reviewers independently: (I) general information was extracted such as first author, publication year, method of patient recruitment, sampling method. (II) Diagnostic techniques: IHC for analysis of cytokines expression on prostatic tissue of PC patients, Western blotting and ELISA methods for blood analysis.
Results
Studies identified
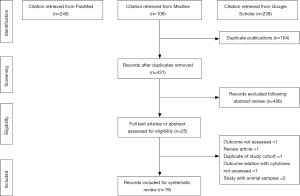
The literature search identified 431 unique citations following the removal of duplicates. Of these 431 citations, 406 were excluded after the first screening stage involving titles and abstract review. In the second screening stage, 25 citations were inspected during a full-text review. Of the 25 studies, 6 articles were excluded for the following reasons: outcome not assessed =1, review article =1, duplication of study cohort =1, outcome relationship with cytokine levels not assessed =1, studies on animal samples =2. A final 19 records were included in this systematic review. The flowchart for the selection process is noted in Figure 1.
Cytokines studies analysis
Of the 19 studies, 9 included analysis of blood samples for cytokines with ELISA, Multiplex immunoassay and immunofluorescence assays methods. In these studies, blood samples were taken prior to and after RT. Of the other 10 articles, prostate tissues biopsies were assessed for cytokines expression with IHC, western blot and real-time PCR techniques.
Possible cytokine expression and correlation with outcomes
A few longitudinal studies have tracked cytokines of interest in blood samples collected during a fractionated course of RT, from pre-treatment baseline through to follow-up (19,21-27). Other studies have also identified cytokine expression using PC tissue biopsies (28-37). The selected studies are divided in two sub group: blood-based biomarkers studies and tissue-based biomarkers studies.
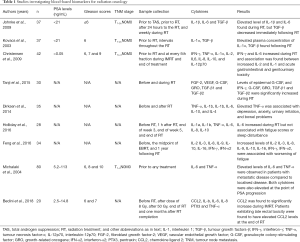
In blood-based biomarkers studies, elevated levels of cytokines have been reported in several types of cancers and have sometimes been correlated with disease progression or poor prognosis. Of the 9 studies, 2 highlighted that elevated plasma cytokines IL-1α, TGF-β, and M-CSF levels were found in patients with PC, compared to healthy individuals (21,22). A study by Michalaki et al. observed that inflammatory cytokines IL-6 and TNF-α levels were higher in patients with metastatic disease compared to patients with localised disease (38). To examine the effect of ADT on cytokine levels, cytokines were measured before and after ADT (prior to RT). Out of 9 studies, 2 informed that cytokines IL-1β, IL-6, fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) levels were elevated in post-ADT blood samples compared to pre-ADT values (21,23).
The effect of RT on circulating cytokine levels was examined before, during and after RT. Of 9 studies, 7 identified altered cytokine levels in post-RT blood sample compared to pre-RT blood samples (19,21-26). We also sought to determine in previous studies if there was a relationship between cytokine levels and patient-reported RT-induced acute and late toxicity. Three of the same 9 studies quantified that elevated cytokines IFN-γ, IL-6, chemokine (C-C motif) ligand-2 (CCL-2), TNF-α and IL-4 levels were associated with increased RT-induced toxicity in PC (19,24,27). The clinical studies investigating blood-based biomarkers are summarized in Table 1.
Full table
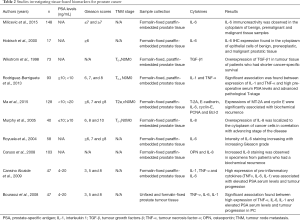
In tissue-based biomarkers studies, numerous earlier studies demonstrated cytokines expression and correlation with clinicopathological characteristics and clinical outcomes of PC patients. Of the 10 studies, 7 revealed intense epithelial cytoplasmic staining in PC cancer biopsies when compared to benign prostate tissue, in those tissues with higher Gleason grade and in patients with elevated PSA levels (28,29,31,33,34,36,37). Four of the same 10 studies, highlighted an association between TNF-α, IL-1, IL-6 and IL-8 overexpression and higher pre-operative serum PSA levels and advanced pathological T stage (31,33,36,37). Furthermore, low intensity for IL-6 expression was detected on tumour tissues with low Gleason grade and the intensity of staining was increasing with increasing Gleason grade (34).
Furthermore, three studies quantified cytokines overexpression on tumour biopsies of PC patients who had shorter cancer-specific survival and biochemical recurrence post-prostatectomy (30,32,35). The studies investigating tissue-based biomarkers are summarized in Table 2.
Full table
Discussion
Though there is strong evidence in the scientific literature regarding the association of cytokines with the development and progression of many cancers, there is limited published data relevant to PC. There is emerging evidence of cytokine involvement in the development and outcomes in PC (38). Michalaki et al. reported that serum IL-6 and TNF-α levels were higher in patients with metastatic disease than in patients with localised disease (38). IL-6 is known to promote the proliferation and metastatic potential of cancer cells (38). This cytokine is a prostate exocrine gene product that interacts with its receptor in prostate cells, regulating proliferation and differentiation, and in PC cell lines activates androgen receptor (38). Moreover, plasma levels of IL-1α, TGF-β, and M-CSF in PC patients were found to be significantly elevated compared to healthy individuals (21,22). The above studies highlighted that IL-6, TNF-α, IL-1α, TGF-β, and M-CSF may be suggested as possible indicators of disease progression.
PC cells are typically androgen-dependent and androgen ablation is the standard systemic therapy for this disease since androgen deprivation induces programmed cell death in normal, hyperplastic, preneoplastic, and malignant prostatic epithelial cells (39,40). ADT associated changes in the hormonal environment strongly affect both host and tumour (41). Because androgens are known to be potent immune modulators, it was of interest to determine the effect of ADT on circulating cytokine levels in PC. To accomplish this, previous studies examined plasma cytokine concentrations in PC patients before and after commencement of ADT. Johnke et al. demonstrated elevated levels of proinflammatory cytokines IL-1β and IL-6 and reduced levels of profibrotic cytokine TGF-β in the post-ADT blood when compared to pre-ADT blood analysis (21). Likewise, a study by Tanji et al. described that ADT significantly decreased the serum levels of FGF-2 and VEGF compared to cytokines concentration in pre-ADT blood (23).
Recently, many studies have focused on the elucidation of clinically useful biomarkers of RT-induced toxicity. Researchers believe that ability to identify a patient’s radiation sensitivity profile could lead to more suitable treatment options, improved loco-regional control and OS (21,22,42). Regarding this, possible association between altered levels of cytokines with RT and the cause of RT-induced toxicity has attained a great discussion in scientific literature (19,21,42,43). Moreover, tumour could also produce multiple amounts of cytokines during RT; therefore, plasma cytokine levels may decrease or increase depending on the tumour response to RT (16,17). Johnke et al. reported that TGF-β, IL-1β, and IL-6 levels were significantly increased during RT compared to the cytokine concentration in blood before RT (21). Additionally, more studies reported, levels of TGF-β1, TGF-β2, IL-6 and IFN-γ were also elevated during RT compared to blood analysis before RT (19,23). Therefore, administration of RT appeared to bring about a noticeable elevation of cytokines levels in PC patients.
In general, the probability of RT-induced toxicity increases as the RT dose increases (44). Cytokines are release in response to ionizing radiation and might play a key role in following RT-induced toxicity (45,46). Some previous studies have reported increased cytokine levels during and after RT and suggested as predictive biomarkers for RT-induced toxicity (47-49). Rubin et al. reported that levels of IL-1, TGF-β, and TNF-α were increased immediately after radiation exposure and that elevated TGF-β levels were associated with increased risk of pulmonary fibrosis (18). Many previous studies in lung cancer also confirmed an association between RT-induced lung toxicity (RILT) and levels of circulatory cytokines (42,50-54). In PC, a study by Christensen et al. confirmed that IFN-γ and IL-6 levels were significantly increased during prostate RT with an associated increase in acute gastrointestinal and genitourinary toxicity (19). Next, CCL2 was significantly increased during IMRT and patients exhibiting late rectal toxicity were found to have elevated levels of CCL2 at the end of RT (27). Dirksen et al. also releveled significant correlations between TNF-α levels and depression (P=0.001), anxiety (P=0.030), urinary irritative (P=0.046), and bowel problems (P=0.007) and between IL-4 levels and urinary irritative symptoms (P=0.035) (24). In above studies, scientists believe that inflammatory cytokines could be predictive biomarkers of RT-induced toxicity.
GS, pre-operative serum PSA and pathologic T stage, alone or in combination, are the most significant prognostic markers for biochemical recurrence (55). However, the accuracy of prediction could be improved by introducing new biomarkers into clinical practice. Rodríguez-Berriguete et al. described that patients with overexpression of TNF-α on tumour tissue had poor clinical outcomes (31). Some previous studies also revealed an association between TNF-α, IL-1, IL-6 and IL-8 overexpression and higher pre-operative serum PSA levels and advanced pathological T stage (31,33,36,37). Furthermore, low intensity was detected for IL-6 expression on tumour tissues with low Gleason grade and the intensity of staining was increasing with increasing Gleason grade (34). There was also a correlation found between OPN, IL-6, TGF-β1, MT-2A, cyclin-E expressions and biochemical recurrence and shorten cancer-free survival (30,32,35). The discovery of new potential biomarkers may provide great benefit to the practice of PC and RT that correlate with side effects and clinical outcomes. However, several assessments of validated biomarkers may be needed to treat PC patients with the highest degree of accuracy and specificity. They may also permit for adaptive RT and re-planning the PC patient based on biomarker endpoints.
Limitations
The studies reviewed used methods of varying quality and standardisation to determine cytokines expression in blood and on the tumour biopsy of PC patients receiving RT. These variable methods are likely to contribute to inconsistency in findings. Sample sizes varied broadly between studies, with one study utilising minimum 17 participants (29), and a study maximum having 128 (31). Regarding significant statistics, it has been recommended as a general rule of thumb in order to obtain adequate data and error sizes that the sample size is at least 42 participants (19). In this systematic review, 9 studies were identified with sample sizes of less than 42.
Conclusions
Our systematic review identified that cytokines levels were directly correlated with the extent of the disease. Above studies also confirmed higher levels of cytokine in patients who were treated with ADT. Moreover, elevated levels of cytokine in PC patients were noticed after immediate exposure to RT and association with RT-induced acute/late toxicity. Thus, cytokine concentration in patients’ blood could be predictive biomarkers for RT-induced toxicity. Above studies also identified overexpression of cytokines on tumour biopsies and there was an association with shortening cancer-specific survival and biochemical recurrence. Therefore, according to above studies, overexpression of cytokines on tumour tissue may serve as independent predictors biomarkers for clinical outcomes of PC patients.
Acknowledgments
The study was supported by funds from the College of Health and Human Sciences, Charles Darwin University, Australia for publication charges.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rebbeck TR. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin Radiat Oncol 2017;27:3-10. [Crossref] [PubMed]
- Shin HR, Masuyer E, Ferlay J, et al. Cancer in Asia - Incidence rates based on data in cancer incidence in five continents IX (1998-2002). Asian Pac J Cancer Prev 2010;11 Suppl 2:11-6. [PubMed]
- Kvale R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst 2007;99:1881-7. [Crossref] [PubMed]
- Feletto E, Bang A, Cole-Clark D, et al. An examination of prostate cancer trends in Australia, England, Canada and USA: Is the Australian death rate too high? World J Urol 2015;33:1677-87. [Crossref] [PubMed]
- . . Available online: https://atlas.cancer.org.auAustralian Cancer Atlas, Cancer Council Queensland, Queensland University of Technology. 2018.
- Zhang X, Condon J, Dempsey K, et al. Cancer in the Northern Territory 1991–2010: Incidence, mortality and survival. Northern Territory, Department of Health, 2014.
- Wu CT, Chen MF, Chen WC, et al. The role of IL-6 in the radiation response of prostate cancer. Radiat Oncol 2013;8:159. [Crossref] [PubMed]
- Harle LK, Maggio M, Shahani S, et al. Endocrine complications of androgen-deprivation therapy in men with prostate cancer. Clin Adv Hematol Oncol 2006;4:687-96. [PubMed]
- Basaria S, Lieb J 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779-86. [Crossref] [PubMed]
- Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9 Suppl 1:S3-8. [PubMed]
- D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [Crossref] [PubMed]
- Deorukhkar A, Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol 2010;80:1904-14. [Crossref] [PubMed]
- Steiner GE, Newman ME, Paikl D, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 2003;56:171-82. [Crossref] [PubMed]
- Poutahidis T, Rao VP, Olipitz W, et al. CD4+ lymphocytes modulate prostate cancer progression in mice. Int J Cancer 2009;125:868-78. [Crossref] [PubMed]
- George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res 2005;11:1815-20. [Crossref] [PubMed]
- Rube CE, Palm J, Erren M, et al. Cytokine plasma levels: reliable predictors for radiation pneumonitis? PLoS One 2008;3:e2898. [Crossref] [PubMed]
- Siva S, MacManus M, Kron T, et al. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS One 2014;9:e109560. [Crossref] [PubMed]
- Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 1995;33:99-109. [Crossref] [PubMed]
- Christensen E, Pintilie M, Evans KR, et al. Longitudinal cytokine expression during IMRT for prostate cancer and acute treatment toxicity. Clin Cancer Res 2009;15:5576-83. [Crossref] [PubMed]
- Oliveira MA. BJCVS/RBCCV and Endnote. Rev Bras Cir Cardiovasc 2015;30:127. [Crossref] [PubMed]
- Johnke RM, Edwards JM, Evans MJ, et al. Circulating cytokine levels in prostate cancer patients undergoing radiation therapy: influence of neoadjuvant total androgen suppression. In Vivo 2009;23:827-33. [PubMed]
- Kovacs CJ, Daly BM, Evans MJ, et al. Cytokine profiles in patients receiving wide-field + prostate boost radiotherapy (xRT) for adenocarcinoma of the prostate. Cytokine 2003;23:151-63. [Crossref] [PubMed]
- Tanji N, Kikugawa T, Ochi T, et al. Circulating Cytokine Levels in Patients with Prostate Cancer: Effects of Neoadjuvant Hormonal Therapy and External-beam Radiotherapy. Anticancer Res 2015;35:3379-83. [PubMed]
- Dirksen SR, Kirschner KF, Belyea MJ. Association of symptoms and cytokines in prostate cancer patients receiving radiation treatment. Biol Res Nurs 2014;16:250-7. [Crossref] [PubMed]
- Holliday EB, Dieckmann NF, McDonald TL, et al. Relationship between fatigue, sleep quality and inflammatory cytokines during external beam radiation therapy for prostate cancer: A prospective study. Radiother Oncol 2016;118:105-11. [Crossref] [PubMed]
- Feng LR, Wolff BS, Lukkahatai N, et al. Exploratory Investigation of Early Biomarkers for Chronic Fatigue in Prostate Cancer Patients Following Radiation Therapy. Cancer Nurs 2017;40:184-93. [Crossref] [PubMed]
- Bedini N, Cicchetti A, Palorini F, et al. Evaluation of Mediators Associated with the Inflammatory Response in Prostate Cancer Patients Undergoing Radiotherapy. Dis Markers 2018;2018:9128128. [Crossref] [PubMed]
- Milicevic N, Mrcela M, Galic J, et al. Expression of proinflammatory cytokine interleukin-6 in tissue samples of human prostate obtained by needle biopsy. Pathol Res Pract 2015;211:865-70. [Crossref] [PubMed]
- Hobisch A, Rogatsch H, Hittmair A, et al. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol 2000;191:239-44. [Crossref] [PubMed]
- Wikstrom P, Stattin P, Franck-Lissbrant I, et al. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998;37:19-29. [Crossref] [PubMed]
- Rodríguez-Berriguete G, Sánchez-Espiridión B, Cansino JR, et al. Clinical significance of both tumor and stromal expression of components of the IL-1 and TNF-alpha signaling pathways in prostate cancer. Cytokine 2013;64:555-63. [Crossref] [PubMed]
- Ma D, Zhou Z, Yang B, et al. Association of molecular biomarkers expression with biochemical recurrence in prostate cancer through tissue microarray immunostaining. Oncol Lett 2015;10:2185-91. [Crossref] [PubMed]
- Murphy C, McGurk M, Pettigrew J, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res 2005;11:4117-27. [Crossref] [PubMed]
- Royuela M, Ricote M, Parsons MS, et al. Immunohistochemical analysis of the IL-6 family of cytokines and their receptors in benign, hyperplasic, and malignant human prostate. J Pathol 2004;202:41-9. [Crossref] [PubMed]
- Caruso DJ, Carmack AJ, Lokeshwar VB, et al. Osteopontin and interleukin-8 expression is independently associated with prostate cancer recurrence. Clin Cancer Res 2008;14:4111-8. [Crossref] [PubMed]
- Cansino Alcaide JR, Vera San Martin R, Rodriguez de Bethencourt Codes F, et al. Prostatic specific antigen (PS), pro-inflammatory cytokines, and prostatic pathology (benign prostatic hyperplasia and cancer). Relationship with malignancy. Arch Esp Urol 2009;62:359-66. [PubMed]
- Bouraoui Y, Ricote M, Garcia-Tunon I, et al. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev 2008;32:23-32. [Crossref] [PubMed]
- Michalaki V, Syrigos K, Charles P, et al. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 2004;90:2312-6. [Crossref] [PubMed]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001;1:34-45. [Crossref] [PubMed]
- Montironi R, Mazzucchelli R, Lopez-Beltran A, et al. Mechanisms of disease: high-grade prostatic intraepithelial neoplasia and other proposed preneoplastic lesions in the prostate. Nat Clin Pract Urol 2007;4:321-32. [Crossref] [PubMed]
- Saylor PJ, Kozak KR, Smith MR, et al. Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. Oncologist 2012;17:212-9. [Crossref] [PubMed]
- Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol 2007;17:89-98. [Crossref] [PubMed]
- Chen Y, Williams J, Ding I, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol 2002;12:26-33. [Crossref] [PubMed]
- Stankovic V, Dzamic Z, Pekmezovic T, et al. Acute and Late Genitourinary Toxicity after 72 Gy of Conventionally Fractionated Conformal Radiotherapy for Localised Prostate Cancer: Impact of Individual and Clinical Parameters. Clin Oncol (R Coll Radiol) 2016;28:577-86. [Crossref] [PubMed]
- Provatopoulou X, Athanasiou E, Gounaris A. Predictive markers of radiation pneumonitis. Anticancer Res 2008;28:2421-32. [PubMed]
- Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets 2013;14:1347-56. [Crossref] [PubMed]
- Arpin D, Perol D, Blay JY, et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J Clin Oncol 2005;23:8748-56. [Crossref] [PubMed]
- Citrin DE, Hitchcock YJ, Chung EJ, et al. Determination of cytokine protein levels in oral secretions in patients undergoing radiotherapy for head and neck malignancies. Radiat Oncol 2012;7:64. [Crossref] [PubMed]
- Zhao L, Wang L, Ji W, et al. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan. Int J Radiat Oncol Biol Phys 2009;74:1385-90. [Crossref] [PubMed]
- Okunieff P, Chen Y, Maguire DJ, et al. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev 2008;27:363-74. [Crossref] [PubMed]
- Hart JP, Broadwater G, Rabbani Z, et al. Cytokine profiling for prediction of symptomatic radiation-induced lung injury. Int J Radiat Oncol Biol Phys 2005;63:1448-54. [Crossref] [PubMed]
- Zhao L, Sheldon K, Chen M, et al. The predictive role of plasma TGF-beta1 during radiation therapy for radiation-induced lung toxicity deserves further study in patients with non-small cell lung cancer. Lung Cancer 2008;59:232-9. [Crossref] [PubMed]
- Evans ES, Kocak Z, Zhou SM, et al. Does transforming growth factor-beta1 predict for radiation-induced pneumonitis in patients treated for lung cancer? Cytokine 2006;35:186-92. [Crossref] [PubMed]
- Stenmark MH, Cai XW, Shedden K, et al. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:e217-22. [Crossref] [PubMed]
- Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. J Cancer 2010;2:1-19. [Crossref] [PubMed]