
Treatment, complications, and outcomes of metastatic disease of the spine: from Patchell to PROMIS
Introduction
The spine is a common location of metastasis for many types of cancer. Some studies have estimated that over 30% of cancer patients will develop metastatic spine disease (1,2). Moreover, as treatments of primary cancers continue to improve, and as cancer patients consequently live longer, the number of patients with spinal metastases will increase. These tumors can have debilitating effects on quality of life and yield complex neurological sequelae as a result of spinal cord compression. The management of spinal metastases continues to evolve, with an expanding importance of surgical management of these patients. This shift in treatment paradigms was spurred by Patchell et al. (3), who demonstrated increased survival with surgery in a landmark randomized controlled trial comparing surgical decompression and radiation vs. radiation alone for compressive spinal metastases. A bourgeoning body of literature on outcomes and complications of surgery for spinal metastases has followed. Surgery is often one component of a multimodal approach to spinal metastases, often coupled with chemotherapy and radiation. This makes it more challenging for outcomes studies to determine the true benefit of individual treatments. There is also a growing body of literature attempting to discern the most effective means by which to assess patient-reported outcomes (PRO) after surgery. This review will summarize recent data on treatment of spinal metastatic disease, complications resulting from surgery, and the evolution of tools used to assess patient reported outcomes in these patients.
Treatment of spinal metastases
To understand treatment of spinal metastases, one must understand the advantages and limitations of medical management, radiotherapy, and surgical treatment. In practice, these modalities are most often used together rather than in isolation.
Medical management
Chemotherapy is usually employed to gain long-term control of spinal metastases. It is less often used as monotherapy unless metastases arise from exceedingly chemosensitive tumors such as lymphoma, seminoma, or neuroblastoma. More frequently, chemotherapy is used as adjuvant therapy with radiotherapy (RT) or with surgery plus RT. The particular chemotherapeutic agent used depends on histology and other tumor-specific characteristics (4-6).
Corticosteroids are also a mainstay of treatment of spinal metastases and are thought to help alleviate vasogenic edema and decrease inflammation, which is especially beneficial in metastases producing spinal cord compression (7). Moreover, steroids may have a direct cytotoxic effect on certain hematological malignancies (myeloma and lymphoma) and, at times, breast cancer (5,6). Exact guidelines for using steroids for spinal metastases have not been established; dosing is generally decided on an ad hoc basis. Past trials have advocated both for using the minimal dose of steroids possible as well as treating with high-dose steroids (particularly when treatment also involves RT) (5,7). Transient improvements in ambulation may be seen after initiating steroids and some patients may remain on steroids long-term to reduce pain (6,7).
Radiotherapy
Traditionally, RT was the predominant treatment modality for spinal metastases. Even today, with mounting evidence supporting the importance of surgical intervention as part of management, RT remains a crucial component of the overall treatment algorithm. The primary goal of RT is generally to reduce pain from metastases, though it can also be used to achieve local control to treat or prevent neurological sequelae from spinal cord compression (6,8).
RT can be administered via conventional external beam radiation therapy (EBRT), stereotactic body radiation therapy (SBRT), or spine-specific stereotactic radiosurgery (SRS), depending on the patient’s treatment goals or other patient-specific factors (4,5,8).
EBRT, the most common form of RT, is regularly the first line of treatment for spinal metastases. A single standard-of-care radiation dose has not been definitively established and is often dependent on the radiosensitivity of the tumor subtype. The most common regimens are 8 Gy in a single fraction, 20 Gy in 5 fractions, and 30 Gy in 10 fractions (8). Past trials examining the efficacy of higher-dose multiple-fraction RT vs. lower-dose single-fraction RT have found mixed results. In a meta-analysis of 25 RT trials for bone metastases, Chow et al. (9) found no significant differences in pain relief rates between 8 Gy single-fraction and 20–30 Gy multiple-fraction RT regimens, though the retreatment rate was higher in the single-fraction group. Higher-dose multiple-fraction RT may produce superior results compared to lower-dose single-fraction RT, but this is also dependent on tumor histology (4,8). EBRT at a dose of 30 Gy in 10 fractions may achieve effective local control in certain metastases such as lymphoma, myeloma, breast cancer, prostate cancer, and germinoma (8). Such a protocol is thus commonly used for spine metastases. However, for radioresistant tumors such as sarcomas, colorectal cancer, malignant melanoma, and renal cell carcinoma, local control rates fail to reach 50% at this dosage (8). For these tumors there is increasing data that larger radiation doses, for instance 40 Gy over 20 fractions, may yield better control rates (4,8).
SBRT and SRS are more novel, targeted RT modalities that can focus a greater amount of radiation on a tumor while reducing radiation toxicity to the surrounding tissues (10,11). SBRT may be given in 2–5 fractions; SRS is often given as only a single dose. The ideal radiation dose and schedule for SBRT and SRS are less universally agreed upon compared to EBRT. Nevertheless, because these forms of RT can deliver a more concentrated dose of radiation, quantities up to three times higher than with EBRT may be given, which results in improved local control (4,10,11). Indeed, when compared to EBRT, this higher achievable radiation dose makes this modality of RT less dependent on the radiosensitivity/radioresistance of a tumor in order to alleviate disease burden (4). When to use SBRT or SRS in lieu of EBRT as first-line treatment is an area under active investigation. Also, under investigation is the role of heavy particle radiotherapy such as proton beam or carbon ion therapy for spinal metastases. Considerably more research is needed on this topic, but the proposed benefits of such radiotherapy would be akin to SBRT or SRS (higher radiation doses with less damage to surrounding tissues) (12).
Surgery
Surgery is now recognized as an integral component of the treatment of spinal metastases. It may be used for several reasons: to address neurologic symptoms of spinal cord compression, to stabilize spinal instability, to reduce pain, to remove epidural tumor before SBRT or SRS, and to provide a histological tumor diagnosis (see Figure 1 for example) (4,6).
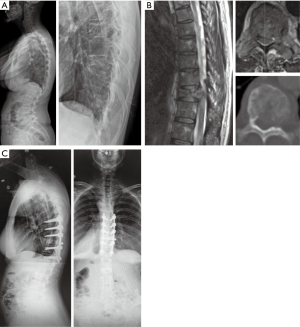
A new emphasis on the importance of surgery for spinal metastases has developed over the past 10–15 years following the seminal 2005 study by Patchell et al. (3) that provided prospective, randomized controlled trial evidence supporting the use of decompressive surgery for such patients. In this trial, patients with spinal metastases were randomized to receive either surgery (n=50) followed by RT or RT alone (n=51). A statistically significant difference in mean survival was seen between the two groups, with patients receiving surgery followed by RT surviving 126 days as compared to 100 days in the group that received RT alone (P=0.033). Additionally, neurological functioning, as determined by ASIA and Frankel scales, was sustained for 566 days on average in the surgery plus RT group vs. 72 days in the group receiving RT alone (P=0.001 for ASIA and P=0.0006 for Frankel). The ability to walk was significantly prolonged in patients who received surgery and RT instead of only RT (122 vs. 13 days; P=0.003), and more patients who were unable to ambulate at the study’s onset regained the ability to walk after surgery and RT compared to solely RT (62% and 19%, respectively; P=0.01). Continence was significantly prolonged (P=0.016) and significantly smaller doses of corticosteroids (P=0.0093) and opioid analgesics (P=0.002) were required in the group that received surgery. Surgery did not lead to prolonged hospitalization. Despite critiques that the trial excluded highly radiosensitive tumors and had a low enrollment rate, it provided convincing evidence of the benefits conferred by surgery. Indeed, because of the notably better outcomes in the surgery group, this study was halted early.
Following Patchell et al. there has been a bourgeoning body of literature that illustrates similar benefits of surgery (6,13-22). A study by Ibrahim et al. (18) helped to support the results of the Patchell trial. This prospective study examined outcomes for 223 patients who underwent surgery with or without radiotherapy and/or chemotherapy for spinal metastases of epithelial origin. After surgery, median survival was 11.7 months, 71% of patients had improved pain, 53% regained or maintained ambulation, and 39% regained urinary continence. As the authors noted, these results compared favorably to the most positive reported outcomes for patients treated by solely RT (23). Falicov et al. (16), in a study of 85 patients who underwent surgery for spinal metastases, concluded that surgery resulted in statistically significantly reduced levels of pain (P<0.00001) and improvements in patient-reported quality of life at 6 weeks (P=0.017), 3 months (P=0.039), and 6 months (P=0.013), with low complication rates. Thomas et al. (24) used the data from the Patchell trial and examined the cost-effectiveness of surgery and RT compared to RT alone for spinal metastases. These researchers determined that surgery plus RT was cost-effective with respect to both the cost per additional day of ambulation and the cost per life-year gained.
Though surgery is now a key aspect of management for spinal metastases, surgery alone is almost invariably palliative. The choice of surgery should depend on a patient’s goals for treatment, the number and location of metastases, and other individual patient characteristics (4-6,22). Surgery should be performed as soon as possible after diagnosis of metastases for optimal outcomes (25). Because its benefits are not immediate, surgery is most effective when a patient’s life expectancy is longer than 8–12 weeks and when the risks are outweighed by the potential improvements (6,26). At times, aggressive resection of metastases via en bloc resection has been shown to achieve high local control rates, but this does not completely preclude future metastases and is most effective for patients with a single metastasis (4-6,21). Moreover, many patients have spinal instability, poor neurological status, or goals of care that obviate en bloc resection, and these procedures are accompanied by relatively high morbidity (6,21). Debulking (when the tumor is resected in portions) or palliative (when the goal is primarily to reduce spinal cord compression) approaches may be used if en bloc resection is not possible (5,19,27). Stabilization can be performed to address spinal instability with or without accompanying decompression. Instrumentation is often depended on for long term stabilization, as there is often a high probability that the spine will not fuse properly around sites of metastases due to large gaps in bony structures, local bone destruction by the tumor, and radiotherapy and/or systemic therapy, which can interfere with the fusion process (see Figure 2 for example). We advocate for the use of bone graft or bone graft substitute to promote fusion, because fusion is possible in these patients despite unfavorable circumstances.
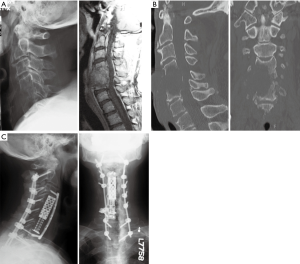
Cement augmentation of vertebral bodies has also demonstrated efficacy, especially in patients with tumors in the anterior section of the spine (4-6,19). The use of cement augmentation through fenestrated screws is a newer trend that may prove similarly useful for mechanical strengthening of spinal constructs in bone that has been pathologically weakened by tumor (28,29). Spinal decompression may be via anterior or posterior approaches, with no consensus in the literature as to which is the superior approach (13,30). Recently, minimally invasive spine surgery has been shown to be a safe and effective technique for decompression and stabilization of the spine that may yield improved functional outcomes and quality of life (31-33).
Since surgery alone likely does not resolve a sufficient amount of tumor burden, it is often used in conjunction with pre- and/or postoperative RT. Research suggests that the timing between surgery and RT should be 1–2 weeks both before and after surgery in order to avoid spurring postsurgical complications (34,35).
Separation surgery is a newer approach by which spinal decompression is performed to create an advantageous opening to the tumor through which SBRT or SRS can deliver a more targeted dose of radiation (8,30,36). There are a number of approaches that can be used for separation surgery, but the transpedicular approach appears to be safe and the most versatile method for a wide range of tumor locations (4). The indications for postoperative RT are still the subject of some debate (37). A prior cost-utility analysis by Furlan et al. (38) concluded that while surgery plus RT was more expensive, this combination also generated superior outcomes to RT alone.
Kyphoplasty or vertebroplasty are more conservative treatments that may be used primarily to manage pain after compression fractures. These treatments can also help provide spine stability in the setting of lytic metastatic disease, particularly in patients with anterior spine instability and those undergoing RT (19).
Complications
Surgery for metastatic spine disease is subject to all the risks of non-oncologic spine surgery, with the added complicating factors of radiation, chemotherapy, coagulopathies, and medical fragility of oncology patients. A thorough understanding of the risks vs. potential benefits is required to make informed decisions about whether an operation is worth pursuing. A recent study of 647 patients undergoing a primary surgery for cervical, thoracic, or lumbar metastatic spine disease estimated a 32% 30-day complication rate after surgery, with 18% undergoing at least one reoperation (39). This is not insignificant, as these complications can potentially decrease quality of life after surgery, which is generally the primary goal. Experiencing a complication was also associated with decreased survival. Factors associated with developing complications were low albumin, additional comorbidities, pathologic fractures, three or more spine levels operated upon, or a combined surgical approach. These risk factors highlight the medical and surgical complexity of these patients that is often not modifiable.
Instrumentation failure is a known complication after surgery for spinal metastases. Abnormal biology in the area where fusion is desired may disrupt bone formation, and this can be further compounded by systemic chemotherapy or local radiation. Therefore, a majority of the forces may continue to be borne by instrumentation when fusion is not possible, due to either bone removal in a decompressive surgery or bone destruction by tumor. Instrumentation failure may become a more common complication as survival increases in these patients. A recent single center cohort study of 159 patients found a 1.9% rate of instrumentation failure requiring reoperation after surgery for metastatic spine disease, with previous radiation to the area being a significant risk (40). The mean survival after surgery of those without instrumentation failure was 17 months, so many patients succumbed to their disease before instrumentation may have failed. Another study of 318 patients at a single center reported a 2.8% instrumentation failure rate requiring reoperation, with chest wall resection and greater than six spinal levels of surgery being risk factors (41), and a third study of 289 patients had a 3.1% reoperation rate for instrumentation failure (42). Along with these factors, a systematic review found that positive sagittal balance and preoperative radiation may contribute to implant failures (43). Kumar et al. proposed a classification of these implant failures, dividing failures into early (<3 months) or late (>3 months), and then further subdividing based on whether the failure is symptomatic (44). The authors did not propose a full treatment algorithm; rather, they noted that there are a significant number of asymptomatic patients that may not need revision despite radiographic failure. They emphasized that surgery should be avoided with these patients to avoid further morbidity. This has been factored into the studies reported here, which only report instrumentation failure requiring reoperation and not asymptomatic failure.
Just as disruption of biology may affect fusion, it may also affect wound healing and increase risk for infection. One study from 2011 reported a very high rate of surgical site infection (SSI) after spine surgery for metastatic disease, up to 8.4% (45). Studies since then have found much lower SSI rates. In a cohort of 159 patients at a single center, 22 of 159 patients required reoperation due to wound dehiscence (6) or wound infection (16,46). Thromboembolic events and increasing number of levels were associated with reoperation in a multivariate model. Quraishi et al. found that infection was the most common reason for reoperation on 384 patients who underwent spine surgery for metastatic disease (42% of second procedures), with an overall infection rate of 4.5% (9/234) (42). A recent systematic review attempted to find risk factors for wound complications and preventative factors (43). After reviewing 40 articles, there was a low level of evidence that preoperative radiation, preoperative neurological deficit, revision surgery, and posterior approaches contribute to wound complications. Plastic surgery soft tissue reconstruction, intrawound vancomycin powder, and percutaneous pedicle screw instrumentation may be protective. As minimally invasive surgery and separation surgery techniques evolve, there may be the potential to further decrease wound complications through the use of tissue sparing techniques. The current literature does not contain a sufficient number of high-quality studies to support that hypothesis at this time (47).
Cost and value are becoming increasingly important factors for measuring outcomes of medical care. To that end, unplanned readmissions is an important metric that can have a significant impact on cost as well as serve as an indicator of morbidity associated with these procedures. Two recent single center studies of 164 and 159 patients looked at readmissions after surgery for metastatic spinal disease (48,49). Thirty-day readmission rates were from 13.8–16.8% and 1-year readmissions were 37.8–47.2%, with approximately 33% due to recurrent disease, 25% due to infection, and 37–43% as a result of medical complications. Prior hospitalization of 15 days and lung metastases were independent risk factors for readmission. Another single center study of 181 patients with both primary and metastatic tumors reported an overall perioperative complication rate or 21.0% with 11.9% 90-day readmissions, costing approximately $20,000 each (50).
Other factors that may affect complication rates include patient age and location of spinal metastases. In a multicenter study of 1,266 patients by the Global Spine Tumor Study group, increased age was associated with increased rates of complications (33.3% >80 years old, 23.9% 70–80 years old, and 17.9% <70 years old), with longer life expectancy in the youngest group and less neurologic recovery in the older group (51). From the American College of Surgeons National Surgical Quality Improvement Program database, cervical location of metastases are associated with highest risk of pulmonary complications and thoracic tumors are associated with highest risk of blood transfusion, whereas lumbosacral tumors have lower odds of perioperative mortality, pulmonary complications, and sepsis (52).
Blood transfusion is also associated with complications. In a single center study, the odds ratio of developing post-operative complications was 2.27 times higher in those who received a transfusion than in those who did not, with an increase in odds ratio of 1.24 per unit transfused (53). Transfusion is not associated with increased overall survival or disease-free survival (54). One method for decreasing transfusion rate is intraoperative cell salvage (IOCS) with a leukocyte depletion filter to remove malignant cells (55). IOCS can replace approximately 50% of the red blood cells lost during surgery while removing tumor cells. Recent advances in selective filtration have been demonstrated to be safe in oncologic surgery in gynecology, hepatobiliary surgery, gastrointestinal surgery, and urology, but there is a paucity of evidence in spinal metastatic disease. There is currently one study that reported lower blood transfusion rates and shorter lengths of stay with use of this technology in surgery for spinal metastases, with no difference in survival or complication rates (56). It will likely take more in depth and larger studies to establish this as a safe and effective technique in musculoskeletal tumor surgery.
Outcomes for spinal metastases
Survival and prognostic factors
Mean survival for spinal metastases is dependent on tumor histology and, depending on the study cited, may range from 51 months for myeloma to 26 months for thyroid cancer to less than 6 months for lung, stomach, esophagus, or pancreatic cancer (57,58). A number of studies (57-63) have attempted to identify prognosticators or use scoring systems to predict post-treatment survival in these patients. A systematic review and meta-analysis by Luksanapruksa et al. (64) identified seventeen prognosticators of poor outcomes across 43 studies, including factors such as age >65 years old, multiple metastases (bone or visceral), ≥3 involved vertebrae, non-ambulatory status before treatment, KPS <70, male gender, and increasing time from cancer diagnosis to surgery. Some commonly cited predictive systems are the Tomita, van der Linden, Bauer, modified Bauer, and revised Tokuhashi (58). These systems all incorporate various combinations of primary tumor characteristics, sites and number of metastases, neurological functioning metrics, or performance scores. The Bauer score and modified Bauer score were considered the most predictive scales (58,59), although recent studies (60,61) posit that a modified Bauer score that incorporates ambulatory status and serum albumin may be a significantly more accurate predictor than the modified Bauer scale alone. Regardless, treatment decisions, including whether or not to perform surgery, should not be guided solely by a prognostic model—they should be grounded in a patient’s symptoms, neurological compromise, and overall fitness.
Quality of life
Until relatively recently, most outcome measures for treatment of spinal metastases focused on survival, recurrence, complications, or measures of function or neurological status (65); less attention was paid to how patients characterized their own health. Consequently, there has been a push towards self-reported assessments of patients’ health and quality of life. Most of these data are accrued by assessing PRO via validated questionnaires (58). Some of these questionnaires are specific for spinal metastatic disease, while others may be designed more for patients with cancer or neck/back pain of any kind. Regarding more commonly used measures of quality of life for spinal metastases, the EuroQol 5-Dimensions (EQ-5D) is designed to elicit responses about patients’ health status in general. The Cancer Quality of Life Questionnaire Core 30 (QLQ-C30) also has been used, though this is a lengthy questionnaire and is not specific for spinal metastases. Because spinal metastases frequently cause neck and back pain, the Neck Disability Index (NDI) and the Oswestry Disability Index (ODI) have been used to assess quality of life, but these scales are also not specific for this patient population. To address these shortcomings, in 2010 the Spine Oncology Study Group created the Spine Oncology Study Group Outcome Questionnaire (SOSG-OQ), which was subsequently validated in 2015 specifically for patients with spinal metastases (65,66). The SOSG-OQ has since been lauded as more useful than older tools for assessing patient-reported quality of life (67).
PROMIS
The ideal PRO measuring tool is a scale that adapts to a patient’s responses in order to generate the most individually-crafted questionnaire. To this end, Computer Adaptive Testing (CAT) can offer a dynamic system that can generate specific questions based on patients’ prior answers to provide the most reliable and complete assessment (68-70). This kind of questionnaire is able to include metrics and incorporate patient characteristics that may not be captured by other PRO measurements such as the EQ-5D or the SOSG-OQ (70).
In 2004, the National Institutes of Health created the Patient-Reported Outcomes Measurement Information System (PROMIS) in order to improve patient self-reporting of symptoms, functioning, and quality of life (68). PROMIS utilizes Item Response Theory (IRT), which is a testing theory that ensures that each individual question is validated for application to the objective of the test as a whole (68). Moreover, PROMIS may be administered by computer to achieve the benefits of CAT. In sum, this ensures that PROMIS is a flexible and comprehensive assessment of PRO that can achieve greater accuracy while measuring a wide range of desired outcomes. PROMIS can also give T-scores for a patient’s reported outcomes, with a standardized mean score of 50 and a standard deviation of 10, which makes the output easier to understand and place into context alongside other patients (68,69).
PROMIS has been studied in patients with non-cancer spine conditions, where it has been shown to take less time to complete, have lower ceiling and floor effects, and to be just as valid when compared to more traditional, “static” spine PRO assessment scales (71). Few studies have used PROMIS specifically for metastatic spine disease. When the SOSG-OQ first was released in 2010, it was recommended by the SOSG for metastatic spine disease given its superior content capacity to measure disease burden (65) compared to other scales at that time. However, since then, PROMIS has often been lauded as the measurement tool of choice for PRO with spine metastases. Studies by Paulino Pereira et al. (67) and Bernstein et al. (72) compared PROMIS to other PRO questionnaires (ODI, NDI, EQ-5D, or SOSG-OQ) to assess pain and physical function for 100 and 51 patients, respectively, with spinal tumors. Both studies concluded that PROMIS was the superior measurement tool for most patient subgroups when compared to nearly every other PRO instrument. The one exception was that SOSG-OQ may be superior to PROMIS for measuring quality of life (67). Findings from Colman et al. (73), concluded that PROMIS was a superior and more responsive tool compared to EQ-5D, NDI, and ODI for assessing quality of life for 27 patients who underwent surgery and eight patients who underwent RT for spine tumors. Another study by van Wulfften Palthe et al. (74) compared PROMIS to number of older PRO measuring systems for patients with sacral spinal tumors, with the researchers recommending that PROMIS surveys be used to assess quality of life in areas such mental health, physical health, pain, gastrointestinal symptoms, sexual function, and social health. Given the comprehensiveness, efficiency, adaptability, and validity of PROMIS, the literature appears to be trending towards this becoming the gold standard for assessing PRO for metastatic spine disease. For measuring quality of life, both PROMIS and the SOSG-OQ may be used reliably.
Conclusions
Spinal metastases are a complex but common manifestation of primary cancers throughout the body. The management of these patients should incorporate individual patient and tumor characteristics, and most likely should involve a multifaceted approach involving radiation, chemotherapy, and surgery. Surgery, even despite its risks and complications, should be used whenever possible for these patients, as it can provide the longest added survival and superior relief of symptoms. There are many different prognostic variable or models that may predict some aspects of patient outcomes. With respect to measuring PRO after surgery, PROMIS currently seems to be the most favorable tool.
Acknowledgments
None.
Footnote
Conflicts of Interest: JM Buchowski receives royalty payments from Globus Medical and K2M and receives institutional fellowship fudning from OMeGA and AOSpine North America. The other authors have no conflicts of interest to declare.
References
- Wong DA, Fornasier VL, Macnab I. Spinal metastases: The obvious, the occult, and the impostors. Spine (Phila Pa 1976) 1990;15:1-4. [Crossref] [PubMed]
- Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop 1995;19:309-11. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Spratt DE, Beeler WH, de Moraes FY, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol 2017;18:e720-30. [Crossref] [PubMed]
- Harel R, Angelov L. Spine metastases: Current treatments and future directions. Eur J Cancer 2010;46:2696-707. [Crossref] [PubMed]
- Rose PS, Buchowski JM. Metastatic disease in the thoracic and lumbar spine: evaluation and management. J Am Acad Orthop Surg 2011;19:37-48. [Crossref] [PubMed]
- Kumar A, Weber MH, Gokaslan Z, et al. Metastatic Spinal Cord Compression and Steroid Treatment. Clin Spine Surg 2017;30:156-63. [Crossref] [PubMed]
- Ejima Y, Matsuo Y, Sasaki R. The current status and future of radiotherapy for spinal bone metastases. J Orthop Sci 2015;20:585-92. [Crossref] [PubMed]
- Chow E, Zeng L, Salvo N, et al. Update on the Systematic Review of Palliative Radiotherapy Trials for Bone Metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Katsoulakis E, Kumar K, Laufer I, et al. Stereotactic Body Radiotherapy in the Treatment of Spinal Metastases. Semin Radiat Oncol 2017;27:209-17. [Crossref] [PubMed]
- Bhattacharya IS, Hoskin PJ. Stereotactic body radiotherapy for spinal and bone metastases. Clin Oncol (R Coll Radiol) 2015;27:298-306. [Crossref] [PubMed]
- Choi D, Bilsky M, Fehlings M, et al. Spine oncology - Metastatic spine tumors. Neurosurgery 2017;80:S131-7. [Crossref] [PubMed]
- Bakar D, Tanenbaum JE, Phan K, et al. Decompression surgery for spinal metastases: a systematic review. Neurosurg Focus 2016;41:E2. [Crossref] [PubMed]
- Bauer HCF, Wedin R. Survival after surgery for spinal and extremity metastases: Prognostication in 241 patients. Acta Orthop Scand 1995;66:143-6. [Crossref] [PubMed]
- Bilsky MH, Laufer I, Burch S. Shifting Paradigms in the Treatment of Metastatic Spine Disease. Spine (Phila Pa 1976) 2009;34:S101-7. [Crossref] [PubMed]
- Falicov A, Fisher CG, Sparkes J, et al. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976) 2006;31:2849-56. [Crossref] [PubMed]
- Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: Results of the prospective multicenter AOSpine study. J Clin Oncol 2016;34:268-76. [Crossref] [PubMed]
- Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. J Neurosurg Spine 2008;8:271-8. [Crossref] [PubMed]
- Kaloostian PE, Yurter A, Zadnik PL, et al. Current paradigms for metastatic spinal disease: An evidence-based review. Ann Surg Oncol 2014;21:248-62. [Crossref] [PubMed]
- Klimo P, Thompson CJ, Kestle JRW, et al. A meta-analysis of surgery vs. conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]
- Vazifehdan F, Karantzoulis VG, Igoumenou VG. Surgical treatment for metastases of the cervical spine. Eur J Orthop Surg Traumatol 2017;27:763-75. [Crossref] [PubMed]
- Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int J Radiat Oncol Biol Phys 1995;32:959-67. [Crossref] [PubMed]
- Thomas KC, Nosyk B, Fisher CG, et al. Cost-effectiveness of surgery plus radiotherapy vs. radiotherapy alone for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys 2006;66:1212-8. [Crossref] [PubMed]
- Quraishi NA, Rajagopal TS, Manoharan SR, et al. Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur Spine J 2013;22:1383-8. [Crossref] [PubMed]
- Daniel JW, Veiga JCE. Prognostic parameters and spinal metastases: A research study. PLoS One 2014;9:e109579. [Crossref] [PubMed]
- Luksanapruksa P, Buchowski JM, Zebala LP, et al. Perioperative Complications of Spinal Metastases Surgery. Clin Spine Surg 2017;30:4-13. [Crossref] [PubMed]
- Barzilai O, McLaughlin L, Lis E, et al. Utility of Cement Augmentation via Percutaneous Fenestrated Pedicle Screws for Stabilization of Cancer Related Spinal Instability. Oper Neurosurg (Hagerstown) 2019;16:593-99. [Crossref] [PubMed]
- Cianfoni A, Distefano D, Isalberti M, et al. Stent-screw-assisted internal fixation: The SAIF technique to augment severe osteoporotic and neoplastic vertebral body fractures. J Neurointerv Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Guarnieri G, Izzo R, Muto M. Current trends in mini-invasive management of spine metastases. Interv Neuroradiol 2015;21:263-72. [Crossref] [PubMed]
- Hamad A, Vachtsevanos L, Cattell A, et al. Minimally invasive spinal surgery for the management of symptomatic spinal metastasis. Br J Neurosurg 2017;31:526-30. [Crossref] [PubMed]
- Toquart A, Graillon T, Mansouri N, et al. Management of spinal metastases by minimcal invasive surgery technique: Surgical principle, indications: A literature review. Neurochirurgie 2016;62:157-64. [Crossref] [PubMed]
- Itshayek E, Yamada J, Bilsky M, et al. Timing of surgery and radiotherapy in the management of metastatic spine disease: A systematic review. Int J Oncol 2010;36:533-44. [PubMed]
- Lee RS, Batke J, Weir L, et al. Timing of surgery and radiotherapy in the management of metastatic spine disease: expert opinion. J Spine Surg 2018;4:368-73. [Crossref] [PubMed]
- Barzilai O, Laufer I, Yamada Y, et al. Integrating evidence-based medicine for treatment of spinal metastases into a decision framework: Neurologic, oncologic, mechanicals stability, and systemic disease. J Clin Oncol 2017;35:2419-27. [Crossref] [PubMed]
- Redmond KJ, Lo SS, Soltys SG, et al. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine 2017;26:299-306. [Crossref] [PubMed]
- Furlan JC, Chan KKW, Sandoval GA, et al. The combined use of surgery and radiotherapy to treat patients with epidural cord compression due to metastatic disease: A cost-utility analysis. Neuro Oncol 2012;14:631-40. [Crossref] [PubMed]
- Paulino Pereira NR, Ogink PT, Groot OQ, et al. Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J 2019;19:144-56. [Crossref] [PubMed]
- Pedreira R, Abu-Bonsrah N, Karim Ahmed A, et al. Hardware failure in patients with metastatic cancer to the spine. J Clin Neurosci 2017;45:166-71. [Crossref] [PubMed]
- Amankulor NM, Xu R, Iorgulescu JB, et al. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J 2014;14:1850-9. [Crossref] [PubMed]
- Quraishi NA, Rajabian A, Spencer A, et al. Reoperation rates in the surgical treatment of spinal metastases. Spine J 2015;15:S37-43. [Crossref] [PubMed]
- Mesfin A, Sciubba DM, Dea N, et al. Changing the adverse event profile in metastatic spine surgery: An evidence-based approach to target wound complications and instrumentation failure. Spine (Phila Pa 1976) 2016;41 Suppl 20:S262-70. [Crossref] [PubMed]
- Kumar N, Patel R, Wadhwa AC, et al. Basic concepts in metal work failure after metastatic spine tumour surgery. Eur Spine J 2018;27:806-14. [Crossref] [PubMed]
- Omeis IA, Dhir M, Sciubba DM, et al. Postoperative surgical site infections in patients undergoing spinal tumor surgery: Incidence and risk factors. Spine (Phila Pa 1976) 2011;36:1410-9. [Crossref] [PubMed]
- Carl HM, Ahmed AK, Abu-Bonsrah N, et al. Risk factors for wound-related reoperations in patients with metastatic spine tumor. J Neurosurg Spine 2018;28:663-8. [Crossref] [PubMed]
- Zuckerman SL, Laufer I, Sahgal A, et al. When less is more: The indications for mis techniques and separation surgery in metastatic spine disease. Spine (Phila Pa 1976) 2016;41 Suppl 20:S246-53. [Crossref] [PubMed]
- Schairer WW, Carrer A, Sing DC, et al. Hospital readmission rates after surgical treatment of primary and metastatic tumors of the spine. Spine (Phila Pa 1976) 2014;39:1801-8. [Crossref] [PubMed]
- Abu-Bonsrah N, Goodwin CR, De la Garza-Ramos R, et al. Readmissions After Surgical Resection of Metastatic Tumors of the Spine at a Single Institution. World Neurosurg 2017;101:695-701.e1. [Crossref] [PubMed]
- Lau D, Chan AK, Theologis AA, et al. Costs and readmission rates for the resection of primary and metastatic spinal tumors: a comparative analysis of 181 patients. J Neurosurg Spine 2016;25:366-78. [Crossref] [PubMed]
- Amelot A, Balabaud L, Choi D, et al. Surgery for metastatic spine tumors in the elderly. Advanced age is not a contraindication to surgery! Spine J 2017;17:759-67. [Crossref] [PubMed]
- Hussain AK, Vig KS, Cheung ZB, et al. The Impact of Metastatic Spinal Tumor Location on 30-Day Perioperative Mortality and Morbidity after Surgical Decompression. Spine (Phila Pa 1976) 2018;43:E648-55. [Crossref] [PubMed]
- Zaw AS, Kantharajanna SB, Maharajan K, et al. Perioperative blood transfusion: does it influence survival and cancer progression in metastatic spine tumor surgery? Transfusion 2017;57:440-50. [Crossref] [PubMed]
- Zaw AS, Kantharajanna SB, Maharajan K, et al. Metastatic spine tumor surgery: does perioperative blood transfusion influence postoperative complications? Transfusion 2017;57:2790-8. [Crossref] [PubMed]
- Kumar N, Chen Y, Zaw AS, et al. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: A systematic review. Lancet Oncol 2014;15:e33-41. [Crossref] [PubMed]
- Elmalky M, Yasin N, Rodrigues-Pinto R, et al. The safety, efficacy, and cost-effectiveness of intraoperative cell salvage in metastatic spine tumor surgery. Spine J 2017;17:977-82. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A Revised Scoring System for Preoperative Evaluation of spine mets. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Leithner A, Radl R, Gruber G, et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J 2008;17:1488-95. [Crossref] [PubMed]
- Wibmer C, Leithner A, Hofmann G, et al. Survival analysis of 254 patients after manifestation of spinal metastases: Evaluation of seven preoperative scoring systems. Spine (Phila Pa 1976) 2011;36:1977-86. [Crossref] [PubMed]
- Goodwin CR, Schoenfeld AJ, Abu-Bonsrah NA, et al. Reliability of a spinal metastasis prognostic score to model 1-year survival. Spine J 2016;16:1102-8. [Crossref] [PubMed]
- Ghori AK, Leonard DA, Schoenfeld AJ, et al. Modeling 1-year survival after surgery on the metastatic spine. Spine J 2015;15:2345-50. [Crossref] [PubMed]
- Eap C, Tardieux E, Goasgen O, et al. Tokuhashi score and other prognostic factors in 260 patients with surgery for vertebral metastases. Orthop Traumatol Surg Res 2015;101:483-8. [Crossref] [PubMed]
- Pointillart V, Vital JM, Salmi R, et al. Survival prognostic factors and clinical outcomes in patients with spinal metastases. J Cancer Res Clin Oncol 2011;137:849-56. (R Coll Radiol). [Crossref] [PubMed]
- Luksanapruksa P, Buchowski JM, Hotchkiss W, et al. Prognostic factors in patients with spinal metastasis: a systematic review and meta-analysis. Spine J 2017;17:689-708. [Crossref] [PubMed]
- Street J, Lenehan B, Berven S, et al. Introducing a new health-related quality of life outcome tool for metastatic disease of the spine: Content validation using the international classification of functioning, disability, and health; On behalf of the spine oncology study group. Spine (Phila Pa 1976) 2010;35:1377-86. [Crossref] [PubMed]
- Janssen SJ, Teunis T, van Dijk E, et al. Validation of the Spine Oncology Study Group—Outcomes Questionnaire to assess quality of life in patients with metastatic spine disease. Spine J 2017;17:768-76. [Crossref] [PubMed]
- Paulino Pereira NR, Janssen SJ, Raskin KA, et al. Most efficient questionnaires to measure quality of life, physical function, and pain in patients with metastatic spine disease: a cross-sectional prospective survey study. Spine J 2017;17:953-61. [Crossref] [PubMed]
- Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007;45:S3-11. [Crossref] [PubMed]
- Brodke DJ, Saltzman CL, Brodke DS. PROMIS for Orthopaedic Outcomes Measurement. J Am Acad Orthop Surg 2016;24:744-9. [Crossref] [PubMed]
- Fidai MS, Saltzman BM, Meta F, et al. Patient-Reported Outcomes Measurement Information System and Legacy Patient-Reported Outcome Measures in the Field of Orthopaedics: A Systematic Review. Arthroscopy 2018;34:605-14. [Crossref] [PubMed]
- Brodke DS, Goz V, Voss MW, et al. PROMIS PF CAT Outperforms the ODI and SF-36 Physical Function Domain in Spine Patients. Spine (Phila Pa 1976) 2017;42:921-9. [Crossref] [PubMed]
- Bernstein DN, Bakhsh W, Papuga MO, et al. An Evaluation of PROMIS in Patients with Primary or Metastatic Spine Tumors. Spine (Phila Pa 1976) 2019;44:747-52. [Crossref] [PubMed]
- Colman MW, Karim SM, Lozano-Calderon SA, et al. Quality of life after en bloc resection of tumors in the mobile spine. Spine J 2015;15:1728-37. [Crossref] [PubMed]
- van Wulfften Palthe ODR, Janssen SJ, Wunder JS, et al. What questionnaires to use when measuring quality of life in sacral tumor patients: the updated sacral tumor survey. Spine J 2017;17:636-44. [Crossref] [PubMed]


