
Management of hemoptysis in patients with lung cancer
Introduction
Hemoptysis related to malignancy is a common and difficult problem to manage. Its presentation can be subtle or dramatic, which poses many challenges to the provider. Cancers of the thoracic cavity account for nearly a quarter of all cases of hemoptysis (1). Here, we will discuss the initial evaluation and diagnostic strategy when assessing a patient with hemoptysis of different severity, related to malignancy. Specific emphasis will be on relevant signs and symptoms, imaging, and the role of bronchoscopy. After identification, the management of minor and massive hemoptysis remains of high clinical importance because of high mortality rate without appropriate treatment (2). Although the role of surgical management is very limited in this patient population, the role of endobronchial and endovascular management will be discussed in detail.
Definition
In general, hemoptysis is defined as expectoration of blood, alone or mixed with mucous, from the lower respiratory tract. By most accounts, minor hemoptysis is considered to be less than 100 mL of blood expectorated in a 24-hour period (3-6) the definition of massive hemoptysis however, remains poorly defined in the literature because the terms used to describe the amount of hemoptysis are inconsistently utilized. Life-threatening or massive hemoptysis is defined by a larger volume of blood, while clinical instability is considered to be a better definition. Reported thresholds for massive hemoptysis in the literature range from 100–1,000 mL in a 24-hour period (6-9). Additionally, other terms such as “major”, “severe”, and “exsanguinating” have been used to define this clinical entity (10). Given lack of accurate reporting of a volume and rate of bleeding from patients and practitioners, volume-based definition of hemoptysis remains inadequate. It is for this reason that “magnitude of effect” may be more adequate to define massive hemoptysis. The “magnitude of effect” is based on clinical consequences of bleeding which may include: airway obstruction, intubation and hypoxemia hypotension, transfusion requirement, single-lung ventilation, and death (11,12).
Pathogenesis
In the setting of malignancy, there are multiple mechanisms of hemoptysis which likely plays a role in the volume and rate of bleeding. Causes of minor and massive hemoptysis include: neovascularization in and relative to the neoplasm, exfoliation of the tumor surface, tumor necrosis, irritation of tumor from cough, erosion of airways into surrounding vascular structures, and iatrogenic bleeding after airway procedure and systemic treatment (13,14).
Epidemiology and natural history
Hemoptysis is a common clinical finding reportedly responsible for 6.8% of outpatient pulmonary clinic visits and 11% of admissions to a hospital pulmonary service (12). It is important to highlight that minor hemoptysis is a much more common presentation of hemoptysis, and massive hemoptysis is relatively rare. In the United States, lung cancer is responsible for approximately 23% of all cases of hemoptysis (1). The highest incidence of bleeding occurs in patients with squamous cell histology, and massive hemoptysis was associated with both cavitation and tumor arising from or eroding through the central airways (15). The presence of cavitation prior to initiation of treatment was also shown to be associated with fatal hemoptysis (16). The source of hemoptysis is often from a bronchial arterial bleed within the tumor, less frequently from tumor erosion into the pulmonary artery (PA), and rarely from systemic arterial rupture (17,18). Of patients who present to the hospital with non-small cell lung cancer (NSCLC), performance status, advanced age, and need for mechanical ventilation were independent predictors of in-hospital morality (17). Although rare; risk of death from hemoptysis has been shown to correlate with the rate of bleeding as well as malignant etiology. In 1987, Corey et al. described the mortality rate of patients with lung cancer and hemoptysis as 59%, and increased to 80% in patients who had both malignancy and hemoptysis >1,000 mL/24 hours (19). The widespread use of bronchial artery embolization (BAE) in the management of hemoptysis has significantly decreased mortality rates. Other factors that affect mortality include evidence of aspiration to the contralateral lung on radiography and hemodynamic instability (10).
Initial evaluation and diagnostic workup
Determining clinical stability remains the first priority when assessing a patient with hemoptysis. Assessment should include evaluation of patient’s ability to protect their airway, manage their secretions and bleeding, and cooperate with additional diagnostic testing. Once this is achieved, detailed history, physical exam, and laboratory data are important initial steps in evaluation of a patient with lung cancer and hemoptysis. Time of onset, estimated expectorated volume, and the rate of bleeding help to risk-stratify patients. Type of thoracic malignancy and location of the tumor also help to define bleeding risk (20); as has been previously stated, squamous cell histology poses the greatest risk of hemoptysis, followed by adenocarcinoma, small cell, and large cell in decreasing incidence (15). Massive hemoptysis is often preceded by weeks of sentinel minor bleeding (15). Sentinel bleeding in a patient with underlying lung cancer should prompt additional evaluation and diagnostic workup. Physical exam should focus special attention to the airway, and rule out extra pulmonary sources of bleeding. Laboratory data including type and cross-matching, complete blood counts with differential, coagulation profile, electrolytes, liver function, and arterial blood gas testing may provide additional clues. Other diagnostic testing should include imaging and or direct visualization, which will be described below.
Chest X-ray remains an important early step in initial assessment because of the widespread availability, ease to obtain, and low cost. Radiographs can help lateralize the location of the problem to a single side or lobe, which then can be used to guide additional testing such as bronchoscopy or angiography (21). In one study, X-ray determined the site of bleeding in only 45% of all-comers with hemoptysis, but included only patients with massive or “large” hemoptysis (22). In a prospective study of 57 patients from the United States with massive and minor hemoptysis, McGuinness and colleagues found only 19% of this cohort had localized radiographic findings (23). In a retrospective study by Herth et al., nearly a quarter of patients with known malignancy and acute hemoptysis were found to have a normal chest X-ray (24). As such, a normal chest X-ray should not exclude disease, but prompt further testing, including computed tomography (CT) and/or flexible bronchoscopy (FB).
There are two methods to evaluate the source of bleeding: CT and FB. While both are comparable in localizing the site of bleeding. CT can be a useful noninvasive tool to locate the cause of bleeding, and create a roadmap to guide additional therapies. Single- and multi-detector row CT scans can provide rapid, safe, and high-quality imaging of the entire thoracic cavity (21). CT aims to provide details about the mediastinum, lung parenchyma, and detect early cancer as a possible source for bleeding. As a consequence of hemoptysis, small airways and alveoli may have a distorted appearance, which may disguise more subtle early cancers. In addition, 2D and 3D image reconstruction of vessels and airway anatomy can be used by interventional radiologists, pulmonologists, and thoracic surgeons to guide invasive interventions. When CT is compared to bronchoscopy in patients with massive hemoptysis, CT is more effective at identifying the cause of bleeding (77% vs. 8%, respectively, P<0.001), while there is no significant difference in their ability to determine the site of bleeding (70% vs. 73%, respectively) (22). In a retrospective review of 270 patients with hemoptysis and normal chest X-ray, malignancy was identified in 26 patients who underwent further testing with CT and bronchoscopy. Of the 26 patients diagnosed with cancer, the underlying cancer was identified by CT scan in 96% compared to only 54% during bronchoscopy (25). This finding was also confirmed in a prospective study of 91 cases of hemoptysis; CT scan identified 34 tumors while bronchoscopy identified only 27 (26). CT also provides valuable information in the assessment for endovascular management (e.g., BAE) (27). While CT scan is more effective in diagnosing the underlying cause of hemoptysis; it requires the patient to have a secure airway for safe travel to a CT scanner. It is noteworthy that in the setting of minor hemoptysis, CT scan is often the most appropriate next step given its superior diagnostic utility and similar ability to localize the site of bleeding. In the setting of massive hemoptysis however, lateralizing the site of bleeding is of paramount importance. Once lateralization and early stabilization are accomplished, focus can be placed on diagnostic investigation of the underlying cause and exact location of hemoptysis. Bronchoscopy can be performed at the bedside and is a necessary tool in order to establish a secure airway. Hence, the order in which diagnostic tests and procedures are performed should be tailored based on patient’s presentation, minor vs. massive hemoptysis, and access to equipment.
Initial approach
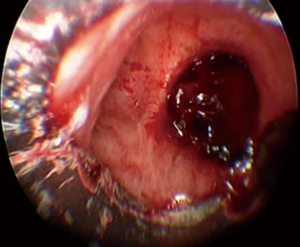
The initial approach to massive hemoptysis should always begin with airway management and hemodynamic stabilization. Once stabilized, localizing the side of bleeding is critical in order to protect the non-bleeding lung. In the case of malignancy related hemoptysis, knowing the site of the primary tumor is key and because this is the likely source of hemorrhage; metastatic disease with bilateral lung involvement may be more challenging as there may be many potential bleeding sites. Once the bleeding side is established, it is important to place the patient in a lateral decubitus position with the bleeding side down (Figure 1). This uses gravity to help prevent spillage or aspiration of blood into the unaffected lung (27). Bilateral intra-alveolar infiltrates may be suggestive of “spillage” of blood into the contralateral non-bleeding lung and may indicate impending respiratory collapse (28).
Pre-procedural preparedness
A pulmonologist’s role in the evaluation of lung cancer has evolved significantly over the past 2 decades, and mere assistance in diagnosing the type of tumor has long been replaced with direct involvement in mediastinal staging, using endobronchial ultrasound guidance to localize and biopsy lymph nodes (29). For this reason, bronchoscopy is a commonly required procedure in the primary stages of lung cancer management.
Prior to any bronchoscopic procedure, it is important to perform a thorough review of a patient’s medical history, laboratory results, and medications. Disease states or medications causing abnormal coagulation factors, or platelet abnormalities should be identified. Anticoagulant medications should be held for the appropriate period of time and reversal agents employed if necessary. Consensus guidelines suggest platelet counts of >50,000 for transbronchial lung biopsy (30). Herth et al. established there was no increased bleeding risk with the continuance of aspirin during transbronchial lung biopsy (24). On the other hand, Ernst et al. found that clopidogrel, with or without aspirin, had significant higher bleeding risk in patients and recommended discontinuation prior to bronchoscopy with biopsy (31). There is insufficient data in regards to bleeding risk and the newer generation anti-platelet medications, but it is important to recognize the potential increased risk of bleeding and hold medications when possible. Uremia can also affect platelet function and should be recognized prior to any procedure. Management with uremic patients by prompt hemodialysis and administration of desmopressin have been shown to reduce the rate of bleeding in those with blood urea nitrogen (BUN) >30 mg/dL (32). Attention to these factors is important to minimize bleeding complications.
Management of hemoptysis
When dealing with hemoptysis in the setting of malignancy, the severity of hemoptysis dictates the next step in management. While diagnostic work up is the focus of management in the setting of minor hemoptysis, airway stabilization is of utmost importance in the setting of massive hemoptysis. Defining the magnitude of effect is of paramount importance during the first encounter for hemoptysis. While differentiating minor from massive hemoptysis is a simpler task, the threshold to aggressive airway management in the setting of moderate vs. massive hemoptysis is less clear. Patient ability to clear airway secretions with an effective cough, in addition to underlying lung disease and pulmonary function test, play a vital role.
Establishing a secure airway
While the approach and management of massive hemoptysis is often outlined as a stepwise approach, these steps often need to occur simultaneously in order to successfully manage this life-threatening condition. The decision of whether to intubate or not should occur rapidly. Significant shortness of breath, inability to handle the amount of hemoptysis or secretions, poor gas exchange and/or worsening hypoxemia, hemodynamic instability, or rapid ongoing hemoptysis are all indications for intubation (18,33).
When choosing an endotracheal tube (ETT), it is important to consider the size of the tube and single lumen vs. double lumen ETT (DL-ETT). The fastest and preferred method of intubation is with a large bore single lumen ETT at least ≥8 mm inner diameter, and ideally 8.5–9 mm inner diameter. The large inner diameter allows for the use of a therapeutic bronchoscope and other tools such as bronchial blockers (10). Although, DL-ETT may seem to be a logical choice in the setting of massive hemoptysis to allow single lung ventilation, there are several downsides that make DL-ETTs a poor choice in the setting of life-threatening hemoptysis. First, they require placement and management by expert practitioner. Furthermore, the inner working channels are very small and can occlude with clots, and the small lumen diameter do not permit the passage of larger flexible bronchoscopes to facilitate suction of obstructive clots. Right or left main-stem intubation using bronchoscopic guidance with a large bore single lumen ETT is preferred over a DL-ETT (10).
In the setting of a patient with an ETT, we recommend immediate FB with a therapeutic bronchoscope to clear the airways of clots, allowing for adequate gas exchange and ventilation. This will also allow for airway inspection and localization of the bleeding source. Once the airways are free of blood clots and the bleeding source has been identified, mainstem intubation of the nonbleeding lung may be considered to avoid aspiration of blood. It should be cautioned that during right mainstem intubation, the ETT cuff will occlude the right upper lobe (RUL) bronchus and may further impair gas exchange (27).
Localizing the bleeding source
Minor hemoptysis secondary to malignancy can be investigated with CT and FB after detailed history and physical examination has been completed. Though minor hemoptysis is often managed in the inpatient setting, it can occur in the outpatient setting as well. While the role of FB in minor hemoptysis is limited, as discussed above, it may add additional value in the diagnostic work up when endobronchial or endotracheal tumor erosion is suspected. For example, slow oozing of hypervascular endo-luminal tumors can be detected using FB.
In comparison to minor hemoptysis, identifying and lateralizing the bleeding lung is a vital step in the management of massive hemoptysis. In the case of malignancy, a prior knowledge of tumor site may help in investigating the side of bleeding. However, hemoptysis is the primary presenting sign of malignancy in 7–35% of cases (1,2,13,34); thus, the cause and location of bleeding might be unknown at presentation. Again, the role of FB in lateralization with the intent of stabilizing in patients with massive hemoptysis should be reemphasized (18).
Bronchoscopy has been shown to successfully identify the site (and not necessarily the location) of hemorrhage in 73–93% of episodes of massive hemoptysis (4,22,35). It allows for immediate lateralization and emergent endobronchial management, including airway clearance, unilateral intubation, or endotracheal intubation and mainstem balloon occlusion of the hemorrhaging lung. Furthermore, FB may be beneficial in patients with bilateral lung abnormalities making radiographic localization challenging (27).
Isolation of the hemorrhaging lung and airway
Bronchial blockers
Bronchial blockers should be considered in the setting of rapid, large volume hemoptysis that persists despite conservative measures such as iced saline. Bronchial blockade prevents the aspiration and contamination of blood in the contralateral lung, ultimately allowing for adequate gas exchange and stabilization of the patient. This provides sufficient time for further diagnostic testing and more definitive management. Bronchial blockade can also result in tamponade, clot formation and temporary hemostasis (36).
Several commercially available bronchial blockers such as Arndt blocker, Cohen Flexitip blocker, Fuji Uniblocker, and the EZ Blocker may be used (37,38). A bronchial blocker consists of a long flexible catheter (4.5–9 F, 65–78 cm length) that is inserted through the ETT, usually with bronchoscopic guidance to the bleeding airway. The distal end of the catheter contains a cuff that can be inflated in the main stem or lobar branches. Since bronchial blockers are prone to dislodgement, they are usually placed in a large airway (e.g., left main bronchus, right main bronchus or bronchus intermedius). An adapter at the proximal end of the ETT allows separate ports for entry of the blocker, bronchoscope and attachment of ventilator tubing simultaneously (39). The use of a bronchial blocker is a temporizing measure often used in the first 48–72 hours of hemoptysis; daily FB should be performed to monitor for bleeding as well as the position of the blocker (40). Leaving the blocker in place for longer durations increases the risk of post-obstructive atelectasis, pneumonia, compression trauma and necrosis of the bronchial wall mucosa (27).
PA catheters have been used in the past to manage massive hemoptysis. The advantage of using a PA catheter is that the smaller catheter can be deployed and inflated distally into smaller bronchi and the inflation pressure can be measured and altered according to the size and location of the bronchus where the PA catheter is deployed. In a case series of three non-oncological patients (41) this technique was used to block segmental or sub segmental bronchi with an inflation pressure of 30–50 mmHg. The method was successful in controlling massive hemoptysis in all three cases within 48 hours. However, this technique is rarely employed because blockade of a larger airway is often necessary as the exact bleeding source cannot usually be identified.
Therapeutic measures
Conservative management of massive hemoptysis is thought to offer only temporary therapeutic effect with 50–100% recurrence rate in the absence of more definitive measures (42-47). There is limited literature on the role of conservative management of minor hemoptysis.
BAE, as well as endobronchial treatment such as ablation, tumor debulking, and surgery, are among the acceptable therapeutic options, each with varying degree of success.
Airway isolation with bronchial blockers and endobronchial use of iced saline and vasoactive agents are among the conservative methods of hemoptysis management. In recent years, other adjunctive therapy, such as tranexamic acid (TA) has been used, primarily in the setting of minor to moderate hemoptysis.
Bronchoscopic interventions
Endobronchial therapeutic installation
Endobronchial instillation of various agents is used in an attempt to obtain hemostasis, particularly when the source of bleeding is beyond reach of the bronchoscope (48,49). In the 1970s, Conlan et al. (50) used ice-cold saline irrigation in 12 patients with massive hemoptysis defined as >600 mL of blood within 24 hours. In their study, irrigation with 4 °C normal saline in 50 mL aliquots was performed using rigid bronchoscopy. Each patient underwent 300–750 mL of ice-cold saline lavage to the bleeding site. Hemostasis was achieved in all 12 patients. Transient bradycardia during irrigation was noted in one patient, and two patients experienced recurrent hemoptysis in the same hospitalization. All patients were discharged from the hospital without additional intervention and three patients with bronchiectasis had followed up elective surgical resection. Though Conlan’s results in treating massive hemoptysis with iced saline seems promising in benign conditions—none of the patients in this series had a thoracic malignancy. Zamani et al. (51) described two patients who were effectively treated with intralesional hexacapron insufflation to control bleeding. A Japanese by Tsukamoto et al. (48) showed good experience with endobronchial fibrinogen—thrombin infusion resulting in hemoptysis cessation in 31/33 patients, again, none of the patients had a thoracic malignancy. Epinephrine and norepinephrine have been used in several cases of hemoptysis following transbronchial lung biopsy to control bleeding, however the literature reports many different doses and dilutions of epinephrine (52-56). Mall et al. reported the use of 1:1,000 epinephrine on 21 patients undergoing endobronchial biopsies in an average dose of 0.12 mg of epinephrine injected endobronchially after the biopsy with acceptable results (57). Cardiac arrhythmia has been noted with epinephrine doses as low as 0.1 mg and caution must be exercised with endobronchial instillation. Due to the dilution effect of these agents within the airway, the role of vasoactive agents in the setting of massive hemoptysis is questionable at best. Valipour et al. (58) described a method of bronchoscopy-guided topical hemostatic tamponade therapy (THT) in patients with massive hemoptysis. THT was performed on patients with persistent endobronchial bleeding despite wedging, cold saline solution lavage, and regional instillation of epinephrine. The hemostatic agent used was oxidized regenerated cellulose (ORC), which is a sterile, knitted fabric, which swells into a gelatinous mass that aids in the formation of clot after it has been saturated with blood. THT was successfully performed in 98% of patients (56/57) with an immediate cessation of hemoptysis. In this series 20 (35%) patients suffered from cancer related hemoptysis and 13 of them died from their underlying malignancy during the follow-up period. Follow-up bronchoscopic studies in a subgroup of 14 patients showed complete absorption of the material used for THT with no histological evidence of foreign body tissue reaction.
Given the low risk profile reported with the use of iced normal saline, its availability and ease of use, we recommend to begin with endobronchial 4 °C normal saline to accomplish hemostasis. If epinephrine and norepinephrine is considered, we recommend the use of lower concentrations (1:100,000) in 2 mL aliquots, not exceeding maximum dose of 0.6 mg, close cardiac monitoring and limitation of use in patients with underlying coronary artery disease and cardiac arrhythmias (59). THT using ORC, where available, can be performed when other endobronchial strategies fail.
Argon plasma coagulation (APC)
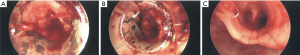
APC is a non-contact method of inducing thermal photocoagulation (44,60,61). This is achieved by using argon, an inert gas, to act as the medium for conduction of electricity. APC photocoagulation induces clot formation and is an effective way to control bleeding in hemoptysis originating from airway wall mucosa (Figure 2). Morice et al. analyzed the use of APC during airway tumor debulking and hemoptysis in sixty patients (43). In this cohort, indications for intervention were hemoptysis (n=31), symptomatic airway obstruction (n=14), and both obstruction and hemoptysis (n=25). Six of these patients had hemoptysis >200 mL per day. There was immediate resolution of hemoptysis in all patients, with no recurrence during a mean follow-up period of about three months.
Endobronchial electrocautery
Endobronchial electrocautery is another effective method to treat hemorrhaging endobronchial tumors. In this method direct electrical energy is transferred to the tissue by contact with electrical energy converted to heat, resulting in coagulation and necrosis. The availability of cautery blades and snares make this a very effective method to debulk endobronchial tumors (40,44).
Laser therapy
Laser, mainly Neodymium-Yttrium Aluminum Garnet (Nd: YAG) emits a focused beam of light that when absorbed by tissues is converted to heat, producing local effects such as photocoagulation, vaporization and necrosis. Laser therapy has been extensively used since the 1970s for management of airway tumors (62,63). Although still primarily used to debulk airway tumors, the photocoagulation effect of laser makes it attractive in the management of hemoptysis (44). Dumon et al. (64) demonstrated effective hemostasis using laser in mild and moderate hemoptysis in over 1,500 patients. They achieved the hemostatic effect using mostly rigid bronchoscope and photo resection with vaporization of underlying tumor using Nd: YAG laser therapy. Encouraging evidence have also been provided by Han et al. in their series of 110 patients treated by 153 Nd: YAG laser sessions for endobronchial tumors (42). Hemoptysis was successfully treated in 40 (77%) of 52 cases. Nine (17%) patients had reduction in the amount of hemoptysis, while the therapy was unsuccessful in three (6%). Consequently, endobronchial laser therapy is considered an effective method in the management of tumors eroding the airway wall with resultant hemoptysis.
All three thermal techniques APC, cauterization and laser when exposed to oxygen might ignite a spark and result in endobronchial fire. Caution must be exercised when using thermal ablative methods and it is prudent to maintain FiO2 of less than 0.4 during treatment application.
Extrathoracic radiotherapy (RT)
In the last 3 decades several studies in advanced primary lung cancer have shown a moderate effect of RT on hemoptysis, though, only palliative (65-67). RT might be a useful palliative modality for hemoptysis secondary to metastatic disease with up to 82% effectiveness (68). As our knowledge and technology have advanced, RT is now seldom used for palliation and only rarely as a treatment modality in hemoptysis.
Brachytherapy
The first application of this modality to treat airway neoplasms was introduced in the 1920s (69,70). A radioactive emitting element mounted on wire is inserted through FB and advanced to the proximity of the neoplasm. Obstructing tumors (even in the setting of partial obstruction) should be de-bulked before introduction of brachytherapy as it can cause inflammation and sequential luminal obstruction. With brachytherapy, mild hemoptysis (71) is alleviated substantially, but the use of this modality in massive hemoptysis is limited.
Endobronchial stents
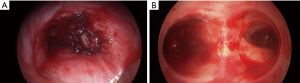
Endo-bronchial stents, primarily silicone, are vastly used for palliative treatment of obstructive airway malignancies (72). Few small series and case reports have found airway stenting to be effective in controlling hemoptysis due to lung cancer (73,74). In most cases, stents were placed after failure of other endobronchial modalities or after laser debulking (Figure 3) (75,76). Most of the stents are deployed using rigid bronchoscopy with isolation of the bleeding bronchial orifice or tumor (69,70,77). When BAE fails, endobronchial stents often in combination with other endobronchial management strategies, are an option in control of hemoptysis.
BAE
When massive hemoptysis or recurrent hemoptysis occurs in a patient with thoracic malignancies, conservative and endobronchial methods are often of limited efficacy (19).
First introduced in 1974, BAE is now widely used, often as a first line and definite therapy in the management of massive hemoptysis (45-47). Arteriogram is performed, commonly via femoral artery cannulation. Embolotherapy of the offending bronchial artery is performed using one of many agents including: gelatin sponge, polyvinyl alcohol (PVA) particles, microspheres, liquid embolic agents such as n-butyl-2-cyanoacrylate, and metallic coil. Each agent has its own advantages and disadvantages, and the choice of agent depends on the operator’s preference and institutional protocols (45).
The success rate of BAE in general, ranges from 60% to 90% (45-47) with immediate bleeding control in cancer related hemoptysis reaching 77–82% (78,79). In a study of 52 patients, Fruchter et al. (45) report immediate BAE technical success of 92% (48/52) within the first 24 hours. Recurrent bleeding occurred in 26 of the 52 patients overall (50.0%); of the malignancy related hemoptysis, 12 of the 16 patients suffered re-bleeding. Most of the recurrence happened during the first month. In a separate study by Fernando et al., malignancy related hemoptysis was associated with a higher likelihood of late bleeding recurrence (47).
Another series by Seki and Shimono (80) evaluated injection of chemotherapy (cisplatin and fluorouracil) to the tumor feeding arteries followed by embolization with HepaSphere or gelatin sponge particles. They report 90% resolution of hemoptysis.
Despite immediate control of bleeding in most malignancy induced hemoptysis, recurrence of malignancy related hemoptysis is common even after BAE. Complications of BAE, although infrequent, include self-limiting chest pain, self-limiting dysphagia from non-target embolization to the esophagus, and transverse myelitis from non-target embolization to the spinal artery (81,82).
Surgery
The role of surgery in hemoptysis has decreased over time and is now reserved for situations when the aforementioned interventions fail. Surgery remains the procedure of choice in the management of massive hemoptysis secondary to iatrogenic PA rupture, chest trauma and aspergilloma resistant to other therapeutic options (83,84). Unfortunately, for a patient with hemoptysis related to thoracic malignancy, due to the tumor stage and disease burden, surgery is rarely an option (85-87).
Other adjunctive therapies
TA is a synthetic lysine analogue with anti-fibrinolytic activity by inhibiting the activation of plasminogen to plasmin and by blocking the action of plasma plasmin (88). In certain cases of hemoptysis, oral or nebulized application of TA might be effective in controlling the bleeding (89-92). Though most reports deal with non-cancer related hemoptysis some small case series have showed a significant effect of inhaled and intravenous TA in malignancy related hemoptysis (93,94).
In a recent double-blind randomized trial in 47 patients, Wand et al. (95) studied the safety and efficacy of inhaled TA vs. placebo in patients with non-massive hemoptysis. Forty-seven patients were randomized to receive TA inhalations (n=25) or normal saline (n=22). TA was associated with a significantly reduced expectorated blood volume starting from day 2 of admission. Resolution of hemoptysis within 5 days of admission was observed in more TA-treated patients than in those receiving placebo (96% vs. 50%; P<0.0005). Mean hospital length of stay was shorter for the TA group (5.7±2.5 vs. 7.8±4.6 days; P=0.046), with fewer patients requiring invasive procedure (0% vs. 18.2%; P=0.041). No side effects were noted in either group throughout the follow-up period. The investigators noted a reduced recurrence rate at 1-year follow-up (P=0.009). It is however noteworthy, that the authors’ definition of massive hemoptysis was expectorated blood >200 mL/24 hours, while most published literature uses a different definition of massive hemoptysis. Hence, it is most reasonable to conclude that in this study, inhaled TA was effective and safe in the setting of hemoptysis of 200 mL/24 hours magnitude. In this series there were only 18 patients with malignancy induced hemoptysis, nine patients were treated with inhaled TA. Unfortunately, no subgroup analysis was performed to assess the efficacy in this group.
Conclusions
Hemoptysis is a common presentation in patients with lung cancer, although massive hemoptysis occurs in only 3% of patients. It is vital to investigate the source of hemoptysis is the setting of malignancy as most massive hemoptysis occurs following sentinel bleed history and should be taken seriously. While the primary focus of minor hemoptysis management includes source identification and treatment in a semi-elective setting, the management of massive hemoptysis requires immediate attention to establishing and securing an airway followed by source identification and treatment. A multidisciplinary approach is required when managing malignancy induced hemoptysis. The literature on the diagnosis and treatment of hemoptysis is primarily from retrospective studies with heterogeneous population including both malignant and non-malignant etiologies and should be applied with care. Future prospective multicenter trials in the setting of malignant hemoptysis are required, to further assess the efficacy of different treatment modalities.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reisz G, Stevens D, Boutwell C, et al. The causes of hemoptysis revisited. A review of the etiologies of hemoptysis between 1986 and 1995. Missouri Medicine 1997;94:633-5. [PubMed]
- Knott-Craig CJ, Oostuizen G, Rossouw G, et al. Management and prognosis of massive hemoptysis: recent experience with 120 patients. J Thorac Cardiovasc Surg 1993;105:394-7. [PubMed]
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003;21:421-35. [Crossref] [PubMed]
- Khalil A, Soussan M, Mangiapan G, et al. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007;80:21-5. [Crossref] [PubMed]
- Amirana M, Frater R, Tirschwell P, et al. An aggressive surgical approach to significant hemoptysis in patients with pulmonary tuberculosis. Am Rev Respir Dis 1968;97:187-92. [PubMed]
- Fartoukh M, Khoshnood B, Parrot A, et al. Early Prediction of In-Hospital Mortality of Patients with Hemoptysis: An Approach to Defining Severe Hemoptysis. Respiration 2012;83:106-14. [Crossref] [PubMed]
- Crocco JA, Rooney JJ, Fankushen DS, et al. Life threatening hemoptysis. Arch Intern Med 1968;121:495-8. [Crossref] [PubMed]
- Hirshberg B, Biran I, Glazer M, et al. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest 1997;112:440-4. [Crossref] [PubMed]
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008;32:1131-2. [Crossref] [PubMed]
- Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis 2017;9:S1069-86. [Crossref] [PubMed]
- Ong TH, Eng P. Life threatening hemoptysis requiring intensive care. Intensive Care Med 2003;29:317-20. [Crossref] [PubMed]
- Dweik RA, Stoller JK. Role of bronchoscopy in life threatening hemoptysis. Clin Chest Med 1999;20:89-105. [Crossref] [PubMed]
- Kvale PA, Selecky PA, Prakash UB, et al. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:368S-403S.
- Cho YJ, Murgu SD, Colt HG. Bronchoscopy for bevacizumab-related hemoptysis. Lung Cancer 2007;56:465-8. [Crossref] [PubMed]
- Miller RR, McGregor DH. Hemorrhage from carcinoma of the lung. Cancer 1980;46:200. [Crossref] [PubMed]
- Ito M, Niho S, Nihei K, et al. Risk factors associated with fatal pulmonary hemorrhage in locally advanced non-small cell lung cancer treated with chemoradiotherapy. BMC Cancer 2012;12:27. [Crossref] [PubMed]
- Razazi K, Parrot A, Khalil A, et al. Severe haemoptysis in patients with nonsmall cell lung carcinoma. Eur Respir J 2015;45:756-64. [Crossref] [PubMed]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000;28:1642-7. [Crossref] [PubMed]
- Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci 1987;294:301-9. [Crossref] [PubMed]
- Salajka F. Occurrence of haemoptysis in patients with newly diagnosed lung malignancy. Schweiz Med Wochenschr 1999;129:1487-91. [PubMed]
- Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. Multi-detector row CT of hemoptysis. Radiographics 2006;26:3-22. [Crossref] [PubMed]
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or life threatening hemoptysis? AJR Am J Roentgenol 2002;179:1217-24. [Crossref] [PubMed]
- McGuinness G, Beacher JR, Harkin TJ, et al. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994;105:1155-62. [Crossref] [PubMed]
- Herth FJ, Becker HD, Ernst A. Aspirin Does Not Increase Bleeding Complications After Transbronchial Biopsy. Chest 2002;122:1461-4. [Crossref] [PubMed]
- Thirumaran M, Sundar R, Sutcliffe IM, et al. Is investigation of patients with haemoptysis and normal chest radiograph justified? Thorax 2009;64:854-6. [Crossref] [PubMed]
- Set PA, Flower CD, Smith IE, et al. Hemoptysis: comparative study of the role of CT and fiberoptic bronchoscopy. Radiology 1993;189:677-80. [Crossref] [PubMed]
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010;80:38-58. [Crossref] [PubMed]
- Thompson AB, Teschler H, Rennard SI. Pathogenesis, evaluation, and therapy for massive hemoptysis. Clin Chest Med 1992;13:69-82. [PubMed]
- Yasufuku K, Nakajima T, Chiyo M, et al. Endobronchial ultrasonography: current status and future directions. J Thorac Oncol 2007;2:970-9. [Crossref] [PubMed]
- Wahidi MM, Rocha AT, Hollingsworth JW, et al. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. A survey of pulmonologists and review of the literature. Respiration 2005;72:285-95. [Crossref] [PubMed]
- Ernst A, Eberhardt R, Wahidi M, et al. Effect of Routine Clopidogrel Use on Bleeding Complications After Transbronchial Biopsy in Humans. Chest 2006;129:734-7. [Crossref] [PubMed]
- Mehta NL, Harkin TL, Rom WN, et al. Should Renal Insufficiency Be a Relative Contraindication to Bronchoscopic Biopsy? J Bronchology Interv Pulmonol 2005;12:81-3.
- Ingbar DH. Massive hemoptysis: Initial management. Available online: https://www.uptodate.com/contents/massive-hemoptysis-initial-management
- Latimer KM. Lung Cancer: Clinical Presentation and Diagnosis. FP Essent 2018;464:23-6. [PubMed]
- Hsiao EI, Kirsch CM, Kagawa FT, et al. Utility of Fiberoptic Bronchoscopy Before Bronchial Artery Embolization for Life threatening hemoptysis. AJR Am J Roentgenol 2001;177:861-7. [Crossref] [PubMed]
- Reisz G. Topical hemostatic tamponade: another tool in the treatment of massive hemoptysis. Chest 2005;127:1888-9. [Crossref] [PubMed]
- Hiebert CA. Balloon catheter control of life-threatening hemoptysis. Chest 1974;66:308-9. [Crossref] [PubMed]
- Cohen E. Pro: the new bronchial blockers are preferable to double-lumen tubes for lung isolation. J Cardiothorac Vasc Anesth 2008;22:920-4. [Crossref] [PubMed]
- Mourisse J, Lerou J. Searching for the ideal endobronchial blocker. Anesthesiology 2013;119:990. [Crossref] [PubMed]
- Colchen A, Fischler M. Emergency interventional bronchoscopies. Rev Pneumol Clin 2011;67:209-13. [Crossref] [PubMed]
- Jolliet P, Soccal P, Chevrolet JC. Control of massive hemoptysis by endobronchial tamponade with a pulmonary artery balloon catheter. Crit Care Med 1992;20:1730-2. [Crossref] [PubMed]
- Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd: YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999;20:123-38. [Crossref] [PubMed]
- Fruchter O, Schneer S, Rusanov V, et al. Bronchial artery embolization for massive hemoptysis: long-term follow-up. Asian Cardiovasc Thorac Ann 2015;23:55-60. [Crossref] [PubMed]
- Chen J, Chen LA, Liang ZX, et al. Immediate and long-term results of bronchial artery embolization for hemoptysis due to benign versus malignant pulmonary diseases. Am J Med Sci 2014;348:204-9. [Crossref] [PubMed]
- Fernando HC, Stein M, Benfield JR, et al. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg 1998;133:862-6. [Crossref] [PubMed]
- Tsukamoto T, Sasaki H, Nakamura H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest 1989;96:473-6. [Crossref] [PubMed]
- Prutsky G, Domecq JP, Salazar CA, et al. Antifibrinolytic therapy to reduce haemoptysis from any cause. Cochrane Database Syst Rev 2012.CD008711. [PubMed]
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980;35:901-4. [Crossref] [PubMed]
- Zamani A. Bronchoscopic intratumoral injection of tranexamic acid: a new technique for control of biopsy-induced bleeding. Blood Coagul Fibrinolysis 2011;22:440-2. [Crossref] [PubMed]
- Alraiyes AH, Alraies MC, Machuzak MS. Q. Does massive hemoptysis always merit diagnostic bronchoscopy? Cleve Clin J Med 2014;81:662-4. [Crossref] [PubMed]
- British Thoracic Society Bronchoscopy Guidelines Committee. a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56 Suppl 1:i1-21. [Crossref]
- Lee P, Mehta AC, Mathur PN. Management of complications from diagnostic and interventional Bronchoscopy. Respirology 2009;14:940-53. [Crossref] [PubMed]
- Ernst A. Introduction to bronchoscopy. New York: Cambridge University Press, 2009:105.
- Parkash UB. Bronchoscopy. Philadelphia: Lippincott-Raven, 1994:238.
- Mall W, Abel H. Topical application of epinephrine during bronchoscopy in barbiturate-halothane-anaesthesia and its influence on cardiac action. Bronchopneumologie 1978;28:311-6. [PubMed]
- Valipour A, Kreuzer A, Koller H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005;127:2113-8. [Crossref] [PubMed]
- Khoo KL, Lee P, Mehta AC. Endobronchial epinephrine: confusion is in the air. Am J Respir Crit Care Med 2013;187:1137-8. [Crossref] [PubMed]
- Kesrouani A, Dabar G, Rahal S, et al. Treatment of tracheal mucoepidermoid carcinoma by argon plasma coagulation during pregnancy. Int Surg 2015;100:927-9. [Crossref] [PubMed]
- Sharifi A, Nazemieh M, Moghadaszadeh M. Supraglottic Hemangioma as a Rare Cause of Recurrent Hemoptysis: A New Treatment Modality with Argon Plasma Coagulation (APC). Tanaffos 2014;13:50-2. [PubMed]
- Kvale PA, Eichenhorn MS, Radke JR, et al. YAG laser photoresection of lesions obstructing the central airways. Chest 1985;87:283-8. [Crossref] [PubMed]
- Wolfe WG, Cole PH, Sabiston DC Jr. Experimental and clinical use of the YAG laser in the management of pulmonary neoplasms. Ann Surg 1984;199:526-31. [Crossref] [PubMed]
- Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest 1984;86:163-8. [Crossref] [PubMed]
- Simpson JR, Francis ME, Perez-Tamayo R, et al. Palliative radiotherapy for inoperable carcinoma of the lung: final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys 1985;11:751-8. [Crossref] [PubMed]
- Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1991;63:265-70. [Crossref] [PubMed]
- Erridge SC, Gaze MN, Price A, et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005;17:61-7. [Crossref] [PubMed]
- Fleming C, Rimner A, Foster A, et al. Palliative efficacy and local control of conventional radiotherapy for lung metastases. Ann Palliat Med 2017;6:S21-S27. [Crossref] [PubMed]
- Yankauer S. Two cases of lung tumor treated bronchoscopically. NY Med J 1992;21:741-2.
- Kernan JD, Carcovaner AJ. Carcinoma of the lung. Arch Surg 1929;18:315-21. [Crossref]
- Murren JR, Buzaid AC. Chemotherapy and radiation for the treatment of non-small-cell lung cancer. A critical review. Clin Chest Med 1993;14:161-71. [PubMed]
- Saji H, Furukawa K, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg 2010;11:425-8. [Crossref] [PubMed]
- Barisione E, Genova C, Grosso M, et al. Palliative treatment of life-threatening hemoptysis with silicone stent insertion in advanced lung cancer. Monaldi Arch Chest Dis 2017;87:781. [Crossref] [PubMed]
- Dalar L, Ozdemir C, Sökücü S, et al. The management of near-fatal hemoptysis with left secondary carinal y stent. Case Rep Pulmonol 2014;2014:709369. [Crossref] [PubMed]
- Brandes JC, Schmidt E, Yung R. Occlusive endobronchial stent placement as a novel management approach to massive hemoptysis from lung cancer. J Thorac Oncol 2008;3:1071-2. [Crossref] [PubMed]
- Chung IH, Park MH, Kim DH, et al. Endobronchial stent insertion to manage hemoptysis caused by lung cancer. J Korean Med Sci 2010;25:1253-5. [Crossref] [PubMed]
- Lee SA, Kim DH, Jeon GS. Covered bronchial stent insertion to manage airway obstruction with hemoptysis caused by lung cancer. Korean J Radiol 2012;13:515-20. [Crossref] [PubMed]
- Garcia-Olivé I, Sanz-Santos J, Centeno C, et al. Predictors of recanalization in patients with life-threatening hemoptysis requiring artery embolization. Arch Bronconeumol 2014;50:51-6. [Crossref] [PubMed]
- Fujita T, Tanabe M, Moritani K, et al. Immediate and late outcomes of bronchial and systemic artery embolization for palliative treatment of patients with nonsmall-cell lung cancer having hemoptysis. Am J Hosp Palliat Care 2014;31:602-7. [Crossref] [PubMed]
- Seki A, Shimono C. Transarterial chemoembolization for management of hemoptysis: initial experience in advanced primary lung cancer patients. Jpn J Radiol 2017;35:495-504. [Crossref] [PubMed]
- Sharma M, Garg M, Ghuman MS. Bronchial artery embolization in chronic pulmonary thromboembolism: A therapeutic dilemma. Lung India 2015;32:624-6. [Crossref] [PubMed]
- Deffebach ME, Charan NB, Lakshminarayan S, et al. The bronchial circulation: small, but a vital attribute to the lung. Am Rev Respir Dis 1987;135:463-81. [PubMed]
- Nakajima T, Yoshino I. Massive Hemoptysis; Clinical Approach and Surgical Treatment. Kyobu Geka 2015;68:665-70. [PubMed]
- Kiral H, Evman S, Tezel C, et al. Pulmonary Resection in the Treatment of Life-Threatening Hemoptysis. Ann Thorac Cardiovasc Surg 2015;21:125-31. [Crossref] [PubMed]
- Rosenzweig KE, Movsas B, Bradley J, et al. ACR appropriateness criteria on nonsurgical treatment for non-small-cell lung cancer: poor performance status or palliative intent. J Am Coll Radiol 2009;6:85-95. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Crawford J, Wheatley-Price P, Feliciano JL. Treatment of Lung Cancer in Medically Compromised Patients. Am Soc Clin Oncol Educ Book 2016;35:e484-91. [Crossref] [PubMed]
- Ng W, Jerath A, Wąsowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339-50. [Crossref] [PubMed]
- Hurley M, Bhatt J, Smyth A. Treatment massive haemoptysis in cystic fibrosis with tranexamic acid. J R Soc Med 2011;104 Suppl 1:S49-52. [Crossref] [PubMed]
- Graff GR. Treatment of recurrent severe hemoptysis in cystic fibrosis with tranexamic acid. Respiration 2001;68:91-4. [Crossref] [PubMed]
- Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999;57:1005-32. [Crossref] [PubMed]
- Bellam BL, Dhibar DP, Suri V, et al. Efficacy of tranexamic acid in haemoptysis: A randomized, controlled pilot study. Pulm Pharmacol Ther 2016;40:80-3. [Crossref] [PubMed]
- Hankerson MJ, Raffetto B, Mallon WK, et al. Nebulized Tranexamic Acid as a Noninvasive Therapy for Cancer-Related Hemoptysis. J Palliat Med 2015;18:1060-2. [Crossref] [PubMed]
- Segrelles Calvo G, De Granda-Orive I, López Padilla D. Inhaled Tranexamic Acid as an Alternative for Hemoptysis Treatment. Chest 2016;149:604. [Crossref] [PubMed]
- Wand O, Guber E, Guber A, et al. Inhaled Tranexamic Acid for Hemoptysis Treatment: A Randomized Controlled Trial. Chest 2018;154:1379-84. [Crossref] [PubMed]


