
All-posterior total en bloc spondylectomy for thoracic spinal tumors
Introduction
The surgical management of malignant and benign aggressive spine tumors is individualized but generally involves local control of the tumor, mechanical stabilization of the spine, neural element protection, and optimization of quality of life. In some cases, a curative, wide-margin algorithm can increase overall survival (1-4). Historically, curettage and intralesional resection with removal of neoplastic tissues in piecemeal fashion were conventional practice, due to the difficulty of the surgical approach and proximity to major vessels and neurologic structures (5,6). However, given an evolved understanding of surgical anatomy, technical development of procedure-specific devices and implants, and a paradigm shift in the surgical oncology of spine tumor management, Enneking-appropriate procedures have been applied with success to a variety of clinical situations (5,7). These can include primary spinal malignancy, oligometastatic malignancy, and even benign aggressive spinal disease (2,3,5,7).
A meticulous understanding of tumor extent according to the Weinstein, Boriani, Biagini (WBB) classification system is helpful in understanding and planning wide-margin surgical treatment of spinal neoplasms. Certain cases may require only limited anterior, posterior, or lateral element resection. However, tumors which occupy a majority of the vertebral body with or without spinal canal extension may require a full deletion of the spinal segmental level(s) in order to achieve adequate margins.
Total en bloc spondylectomy (TES) was first proposed in the 1970s. The original TES case, performed for giant cell tumor of bone, was published by Stener in 1971, and was subsequently popularized by Roy-Camille (8,9). Several technical approaches have been described, including a large all-posterior series by Tomita et al. and an anterior/posterior series by Fidler et al. published as early as 1994 (10-12). The term TES is somewhat of a misnomer, since by definition it involves disruption of the bony neural ring via bilateral pediculotomy and posterior en bloc laminectomy followed by the en bloc vertebrectomy. The rationale behind an all-posterior TES is to accomplish the surgical goals with minimal morbidity. Although comparative data are lacking, the avoidance of a two-stage procedure with full thoracotomy would be theoretically beneficial in perioperative pain levels, pulmonary function, operative time, blood loss, hospital stay, and other variables (11,13-15). It also allows removal of the tumor with the spinal cord in direct visualization.
The purpose of this report is to describe two cases of all-posterior spondylectomy using the recently developed Resegone retractor (K2M, Leesburg, VA, USA), which facilitates an all-posterior resection.
Case presentation
Case 1
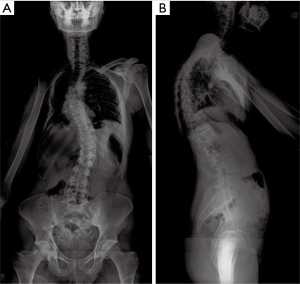
A 30-year-old male with past medical history of adolescent-onset Ewing’s sarcoma of the right upper chest wall treated 23 years ago with surgery, chemotherapy, and thoracic radiotherapy presented to clinic with 8 months of midline upper back pain first noticed after a skiing trip. Of note, the patient had a pre-existing adolescent scoliosis. Long-term sequelae form his treatment included chemotherapy-induced cardiomyopathy, and a post-radiation osteosarcoma to the right clavicle/scapula which was successfully resected along with further adjuvant chemotherapy treatment. He experienced a ten-year disease-free interval before presenting to us. Physical exam was notable for an obvious thoracolumbar scoliosis deformity, hypoplastic right chest, and new bilateral and symmetric deep tendon hyporeflexia. Otherwise the patient was neurologically normal, including a negative Hoffmann sign. Initial radiographs [anteroposterior (AP) and lateral views standing scoliosis] showed evidence of the prior right clavicle and scapular resection, scoliotic deformity with Cobb angle of approximately 30° of both curves and overall coronal and sagittal balance (Figure 1).
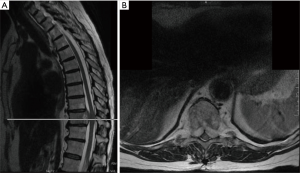
Lumbar and cervical MRI showed mild degenerative changes without significant pathology. However, thoracic MRI and CT scans revealed a lytic lesion and complete collapse of the T7 vertebral body, with soft tissue mass extension into the epidural space at the T6 and T8 bodies (MESSC Scale grade IV, Figure 2) (5). The patient subsequently underwent a percutaneous transpedicular T7 biopsy, which displayed a monotonous fibroblast-like spindle cell proliferation with pleomorphic and hyperchromatic nuclei indicative of a high-grade post-radiation spindle cell sarcoma. Positron emission tomography (PET)/CT scan staging identified a hypermetabolic focus at the T7 vertebral body without evidence of other sites of disease.
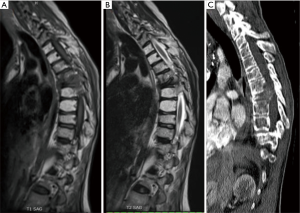
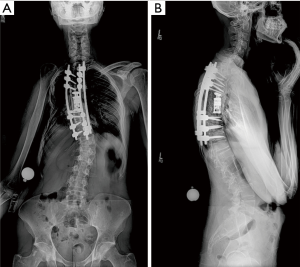
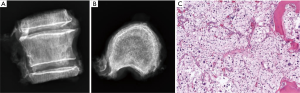
Given the clinical history and exam findings, imaging, histology, recommendation of the multidisciplinary tumor board, and patient preference, the decision was made to proceed with a multilevel en bloc spondylectomy with the goal of a curative resection. Induction chemotherapy was performed. A restaging thoracic MRI just prior to the en bloc spondylectomy revealed mild tumor progression with further extension into the T6 and T8 bodies with some increase in cord stenosis (Figure 3). Two months after initial presentation, this patient underwent an all-posterior T6–8 three-level en bloc spondylectomy, reconstruction with an expandable titanium device, and T3–11 posterior spinal instrumentation (Figure 4). The resection was facilitated using the Resegone (K2M) retractor for both the cephalad and caudad cuts. Gross and microscopic margins were negative for tumor, with a marginal epidural margin (Figure 5). Uninvolved spinous process, dorsal lamina, and 4–6 lateral ribs constituted the autograft that was utilized to facilitate biologic fusion. Dorsal lamina could be included because no dorsal tumor was present in this zone and thus all available biologic graft was utilized. In cases with any dorsal tumor involvement, dorsal lamina is not used. The autograft is used in combination with supplementary structural and crushed allograft, and demineralized bone matrix. Motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs) were obtained from before and after positioning. Right lower extremity MEPs and SSEPs were found to be markedly decreased at the end of the grafting step and start of the closure. Per protocol, continuous SSEPs were run throughout the case, with intermittent MEPs every 30 minutes or so, or in response to a change in SSEP. In this case, SSEPs were normal throughout the case and during cage placement, with a spontaneous decrease towards the end of the case as noted. MEPs at that time confirmed the change and were also decreased. No compressive or stretch phenomenon could be identified. Blood pressure and oxygenation parameters were found to be optimized. A wake-up test after closure demonstrated minimal motor function in the right lower extremity, with weak responses in the left lower muscle groups. The motor exam returned to normal spontaneously within two hours after surgery. The correlation of physical exam with wake-up test and for the 2–3 hours postop demonstrated that this was not an anesthetic related phenomenon especially given that at the time of the event no paralytics were on board. Postoperatively, the patient did well initially with functional mobilization and independent ambulation but ultimately succumbed to overwhelming pulmonary disease 9 months following his original spondylectomy.
Case 2
A 70-year-old female three years status-post radical resection of a 9 cm × 7 cm, high-grade, undifferentiated pleomorphic sarcoma (UPS) of the left thigh presented to our emergency department with worsening thoracic back pain despite normal radiographs. Her original tumor had been treated with neoadjuvant chemotherapy and radiotherapy. Seven months prior to presentation, removal of three sarcoma-positive lung nodules was completed via video-assisted thoracoscopic resection with further chemotherapy cycling, but she was otherwise thought to be free of disease at the time of presentation. She was neurologically normal aside from 4+ left L4 strength, 1+ patellar and Achilles reflexes bilaterally, and subtle gait instability.
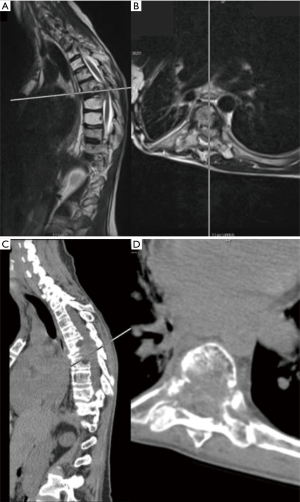
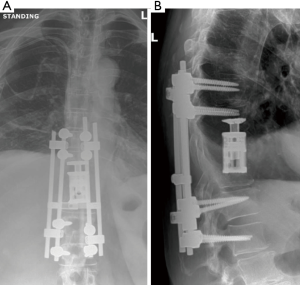
MRI of the thoracic and lumbar spine revealed a lesion involving the T10 vertebral body with an associated soft tissue component posteriorly resulting in compression of the spinal cord (MESSC Scale grade V) (Figure 6). A PET scan was negative. Transpedicular needle biopsy demonstrated high grade pleomorphic sarcoma, consistent with the previous thigh primary. Given the localized disease and early thoracic myelopathy with high grade cord compression, the patient elected to follow the multidisciplinary tumor board recommendation for radical en bloc excision. A radical all-posterior en bloc spondylectomy of T10 with partial T9 and partial T11 spondylectomies were facilitated by the Resegone retractor for both the cephalad and caudad cuts (K2M, Leesburg, VA, USA). Analysis of the removed specimen demonstrated negative margins with a marginal margin at the dural surface and viable high-grade UPS (Figure 7). Reconstruction was performed using an expandable reconstruction cage with posterior instrumentation from T7 to L1 (Figure 8). Biologic fusion was facilitated using rib and uninvolved lamina autograft, supplementary structural and crushed allograft, and demineralized bone matrix. The patient underwent adjuvant stereotactic body radiation and chemotherapy. She is ambulatory with a stable fusion and no evidence of disease 9 months after surgery at the time of this report.
Discussion
Indications for all-posterior approach
An all-posterior TES surgery is primarily designed for patients with a malignant or aggressive benign tumor (10,11) Several factors contribute to a successful all-posterior technique. First, tumor location is critical: an all-posterior TES is best for upper thoracic to thoracolumbar junction spinal tumors since the great vessels are easier to mobilize and nerve root sacrifice is not morbid. Attempting en bloc spondylectomy from an all-posterior technique in the low lumbar spine is technically possible but is dangerous due to the friability and difficulty with blind mobilization of the venous structures, not to mention the morbidity of nerve root sacrifice. Likewise, in the cervical spine, the authors feel that the unique bony, vascular, visceral, and nervous anatomy makes anterior/posterior resections safer than an all-posterior technique. Second, the tumor should be confined mostly to the vertebral body within the chest or retroperitoneum. Preoperative short-spin (T1) MR imaging should be scrutinized to ensure that the tumor does not spread into or invade the adjacent visceral organs, shows little or no adhesion to the vena cava or aorta, and does not involve the segmental arteries, which can create the risk of a vascular avulsion from the aorta. Last, as with any TES case, at least one pedicle and the majority of both laminae should be free of tumor to allow margin-negative opening of the bony neural ring. In cases where this is not possible, tumor contamination is minimized with en bloc posterior element resection via bilateral pediculotomy. Some have termed this a “planned transgression” if in an area that approaches tumor. We use bone scalpel and then bone wax to make the bilateral pedicle cuts. Both serve to minimize bleeding.
Tomita et al. devised a surgical classification system for spinal tumors arising from bone. The anatomic site of the tumor is classified as being in one of five areas: vertebral body [1], pedicle [2], lamina and spinous process [3], spinal canal/epidural space [4], or paravertebral area [5]. These anatomic sites were further stratified into 7 types based on whether the tumor is contained within the the bony cortex (intra-compartmental: types 1–3), outside of the bony cortex (extra-compartmental: types 4–6), or involve multiple nonadjacent vertebra (type 7). It is generally accepted that an all-posterior TES is indicated for types 2, 3, 4, and 5 lesions, relatively indicated for type 1 and 6 lesions, and contraindicated for type 7 lesions (11,13).
Technical tips/tricks and application of a new dural retractor
An all-posterior TES technique is well described and generally includes 3 major portions: a resection of the posterior elements with bilateral costotransversectomy, passage of threadwire saws anterior to the vertebral bodies, and en bloc resection of the anterior column. The traditional approaches were described by Roy-Camille et al., Tomita et al., and others (8,9,11,14,16,17).
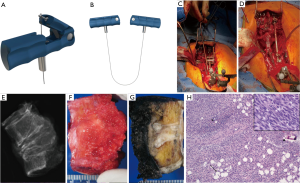
In this series, the authors report on the early experience in North America with the use of a spinal cord protector device as an aide to safe osteotomy (16). This auto-static device consists of a malleable thin lamina of titanium with a single lateral notch on each side. The notches are specifically designed to hold the saw in the correct place and prevent the saw from injuring the thecal sac while guiding it through the posterior body. The device is carefully placed between the thecal sac and the posterior longitudinal ligament and anchored to the rod just prior to initiating the osteotomy. One device can be placed caudal and another cephalad to the planned tumor margins. The thread-wire saws are then oriented within the device(s). With the device in place, the sawing of the bone can be performed without risking pull-through into the cord, while cutting through the desired path in a smooth and parallel fashion.
During preoperative planning and perioperative management, a preoperative angiogram is required to identify critical radiculomedullary arteries if more than 3 levels will be resected. Scrutiny of segmental arteries and their relation to the tumor is critical. If a critical radiculomedullary vessel is identified preoperatively, the authors use a temporary clamping protocol for 10 minutes prior to sectioning. If neuromonitoring signals change, this vessel may need to be preserved along with its attendant nerve root during the resection. Additionally, excellent oxygenation and perioperative blood pressure should be maintained. Mean arterial pressure goals of 80–90 during surgery and for 24 hours postoperatively are advisable. Prophylactic intrathecal drain placement can help drop spinal fluid pressure and increase cord perfusion in very extensive resections.
When conducting costotransversectomy through a posterior approach, the authors perform most of the costotransversectomy with the spinal canal closed to avoid risk to the spinal cord and limit epidural bleeding time. This requires care to not inadvertently avulse nerve roots from the thecal sac. At least 4–5 cm of rib is exposed bilaterally at each segmental level to be resected along with the rib caudal to the resection. Care should be taken to leave the deep periosteum on the pleura to avoid entering the pleura and to allow the potential for rib reconstitution. The rib material is saved for grafting. Intercostal vessels are controlled laterally, and dissected medially to the segmental branch, which allows easy control of segmental vasculature with lateral ligation. This is safer for preserving cord blood supply, and helps avoid difficult to control segmental bleeding near the anterior body deep within the exposure. Laminectomy can be performed using standard high-speed drill technique, osteotomes, or threadwire saws depending on tumor extent. All expendable nerve roots ~1 cm lateral to the thecal sac should be ligated proximal to dorsal root ganglion, and ligatures should not be trimmed. These suture ties are useful in gently retracting the spinal cord later in the case.
Once a clean pleural plane is developed and segmental arteries are identified, an extraperiosteal plane should be developed for the passage of threadwire saws. Subperiosteal planes typically communicate with the disc annulus and make the deep blind dissection more difficult. Gentle, blunt dissection down the vertebral body sidewall bilaterally is facilitated by packing laparotomy sponges deep within the wound as the dissection continues. The authors find early placement of spoon or malleable retractors counterproductive and perform the initial blunt dissection over laparotomy sponges using careful, slow tactile feel with the fingertips, which should communicate in all deep planes. Threadwire saws are passed using Satinsky clamps, and prior to making cuts, deep malleable retractors are placed.
Vertebral resection can be performed with the use of a new malleable rod-mounted spinal cord protector after adequate time is taken for bilateral mobilization of the cord from any epidural tumor and posterior longitudinal ligament, which is removed with the specimen. The malleable retractor passage is facilitated by the included insertion handle, and by slight concave bending of the malleable plate. Once passed, this is pushed against the body and converted to a convex shape. Downward pressure is applied and the retractor is mounted to the posterior instrumentation via the rod. Lateral radiographs confirm the cephalad/caudad location of the cutting slots in accordance to planned cuts. Cephalad and caudal cuts are made, ensuring that the threadwire saws are aligned within the retractor slots. Once the cuts are made, the authors typically cut the saws unilaterally close to the bone and use a pull-through extraction technique. Malleable retractors are removed, and the posterior body wall and posterior longitudinal ligament is cut using the included reverse cutting blade. Specimen extraction is the most risky portion of the procedure. We find this step is facilitated by the included sagittal rod benders, which provide a lordosing moment across the segment and widen the space available for extraction. The specimen can be gently pushed down slightly deeper in the wound, and rotated carefully away from the cord and laterally, usually towards the side of the most extensive tumor involvement. Reconstruction can then commence.
Conclusions
All-posterior TES can be facilitated technically with the use of a posterior, rod-mounted, malleable thecal sac protector. We describe two illustrative cases of its use, and believe that this device can help ensure clean, parallel cuts at the intended level and limit the risk of threadwire saw injuries.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this Case report and any accompanying images.
References
- Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 2010;19:215-22. [Crossref] [PubMed]
- Fisher CG, Saravanja DD, Dvorak MF, et al. Surgical management of primary bone tumors of the spine: validation of an approach to enhance cure and reduce local recurrence. Spine (Phila Pa 1976) 2011;36:830-6. [Crossref] [PubMed]
- Bauer H, Tomita K, Kawahara N, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2002;27:1124-6. [Crossref] [PubMed]
- Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine (Phila Pa 1976) 1997;22:1773-82; discussion 1783.
- Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976) 1997;22:1036-44. [Crossref] [PubMed]
- Bilsky MH, Boland PJ, Panageas KS, et al. Intralesional resection of primary and metastatic sarcoma involving the spine: outcome analysis of 59 patients. Neurosurgery 2001;49:1277-86; discussion 1286-7. [Crossref] [PubMed]
- Chan P, Boriani S, Fourney DR, et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine (Phila Pa 1976) 2009;34:384-91. [Crossref] [PubMed]
- Stener B. Total spondylectomy in chondrosarcoma arising from the seventh thoracic vertebra. J Bone Joint Surg Br 1971;53:288-95. [Crossref] [PubMed]
- Roy-Camille R, Mazel C, Saillant G, et al. Treatment of malignant tumors of the spine with posterior instrumentation. In: Sundaresan N, Schmidek HH, Schiller AL, et al. editors. Tumors of the spine, diagnosis and clinical management. W B Saunders: Philadelphia, PA, 1990:473-87.
- Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy for solitary spinal metastases. Int Orthop 1994;18:291-8. [Crossref] [PubMed]
- Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine (Phila Pa 1976) 1997;22:324-33. [Crossref] [PubMed]
- Fidler MW. Radical resection of vertebral body tumours. A surgical technique used in ten cases. J Bone Joint Surg Br 1994;76:765-72. [Crossref] [PubMed]
- Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci 2006;11:3-12. [Crossref] [PubMed]
- Liljenqvist U, Lerner T, Halm H, et al. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J 2008;17:600-9. [Crossref] [PubMed]
- Sciubba DM, De la Garza Ramos R, Goodwin CR, et al. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur Spine J 2016;25:4080-7. [Crossref] [PubMed]
- Gasbarrini A, Simoes CE, Amendola L, et al. Influence of a thread wire saw guide and spinal cord protector device in "en bloc" vertebrectomies. J Spinal Disord Tech 2012;25:E7-12. [Crossref] [PubMed]
- Charest-Morin R, Fisher CG, Varga PP, et al. En Bloc Resection Versus Intralesional Surgery in the Treatment of Giant Cell Tumor of the Spine. Spine (Phila Pa 1976) 2017;42:1383-90. [Crossref] [PubMed]


