
Restoring the regenerative balance in neuromuscular disorders: satellite cell activation as therapeutic target in Pompe disease
Introduction
Pompe disease is an inherited metabolic disorder due to deficiency in the lysosomal enzyme acid alpha glucosidase (GAA). Pompe disease is characterized by progressive degeneration of skeletal muscle in all patients. In classic infantile patients development of hypertrophic cardiomyopathy (1) and involvement of the central nervous system (2,3) is observed. Enzyme replacement therapy (ERT) extends survival for classic infantile patients and improves motor function, although the heterogeneous response between patients remains challenging (4-6). Together, with the cost of therapy and the burden of the regular infusions for patients and caretakers it is clear that novel treatment strategies are warranted. Such novel therapeutic opportunities include improved ERT, substrate-reduction strategies, the use of chaperone, antisense oligonucleotides, and gene therapy approaches (7,8).
Pompe disease is one of the almost 900 different conditions associated with a muscle wasting phenotype (9). Although different muscle disorders are heterogeneous in disease mechanism and in pattern of progression, these conditions often share a defect in muscle regeneration. Muscle-regenerative pathways are common for all patients with neuromuscular disorders and may offer novel therapeutic opportunities to improve the muscle phenotype across the different diseases. Here, we review muscle regeneration defect(s) in neuromuscular diseases, focusing on Pompe disease. We will also discuss advances in the development of regenerative therapies and the feasibility of using these in the treatment of Pompe disease patients.
Mechanism of the regenerative defect in Pompe disease
Muscle pathology in Pompe disease
Pompe disease is considered a clinical spectrum indicating that the disease can develop at any age and progress at any rate. The onset and progression of disease is roughly correlated with the genotype (10) with classic infantile patients harbouring severe GAA mutations and showing an aggressive course of disease. Deficiency of GAA causes glycogen accumulation that is particularly damaging to skeletal muscle in all patients (11), and to the heart and central nervous system in classic infantile patients (3,12). Skeletal muscles from Pompe disease patients are characterized by vacuolization, irregularly shaped myofibers, and loss of cross-striation (1). During disease progression the lysosomal compartment grows in size and number, replacing healthy cellular content (11). The numerous glycogen-filled lysosomes rupture and release glycogen into the cytoplasm. In the final stages cytoplasmic glycogen has replaced most contractile elements of the muscle cell, resulting in loss of myofiber function. Obviously, glycogen accumulation and lysosomal dysfunction affect other processes that contribute to the pathology, including disturbed macroautophagy (herein referred to as autophagy) and calcium homeostasis, oxidative stress, and mitochondrial abnormalities. Autophagy dysfunction, which is common among lysosomal storage disorders (13), contributes to sarcopenia during aging and will be discussed further below in the context of the muscle regenerative response.
Pathological tissue remodeling, where myogenic tissue is replaced by fat or fibrotic tissue, is a frequent feature of neuromuscular disorders. Adipose deposition of certain muscle groups is observed in most Pompe disease patients at the macroscopic level, and is most prominent in axial muscles, scapular girdle muscles, thigh muscles and specifically tongue (14,15). Pompe disease patients show a typical distribution of affected muscles, with shoulder abductors, abdominal muscles, paraspinal muscles, hip flexors, hip extensors, and hip adductors among the most affected muscles. Hip abductors were affected in more than 80% of all patients (16). The quadriceps femoris was affected in little over half of the patients, while the hand and feet muscle were found to be relatively spared.
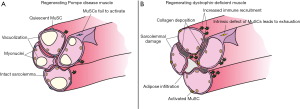
The muscle damage in Pompe disease develops predominantly intracellularly, leaving the sarcolemma and basal lamina largely intact. This is in contrast to the increased sarcolemmal fragility, increased sensitivity for contractile damage, and myofiber rupturing that characterizes dystrophic muscle. Ruptured myofibers recruit immune cells that contribute to pathological deposition of adipose and fibrous tissue in dystrophic muscle. A robust intramuscular immune response to progressing disease is absent in Pompe disease, consistent with maintained sarcolemmal integrity. The ruptured and necrotic myofibers in dystrophic muscle also drive the muscle regenerative response and robustly activate MuSCs. Therefore, the lack of robust sarcolemmal damage in Pompe disease may contribute to satellite cell inactivity, as will be delineated further below. The comparison of Pompe disease and dystrophic muscle illustrates that differences in muscle pathology can have profoundly different effects on muscle regeneration.
The role of satellite cells in muscle regeneration
To understand the clinical consequences of distinct defects in muscle regeneration, we will first discuss the role of skeletal muscle stem cells and muscle regeneration in healthy skeletal muscle. Skeletal muscle can regenerate damage by recruiting the activity of tissue-resident stem cells. Skeletal muscle stem cells (MuSCs) or muscle satellite cells are located at the myofiber periphery underneath the basal lamina (17). In homeostasis MuSCs are quiescent, but become rapidly activated after damage and proliferate to generate a large set of progeny that is capable of regenerating damaged myofibers by fusion (Figure 1). Quiescent MuSCs express high levels of Pax7, a master regulator of postnatal myogenesis. Upon activation MuSCs downregulate Pax7 and upregulate the determination factor MyoD (18) that drives terminal myogenic differentiation. Part of the activated MuSCs return to quiescence in a process called self-renewal (19) to replenish the stem cell pool and secure long-term regenerative potential. The activated MuSCs proliferate and progress to become a population of myogenic progenitors that eventually start expressing myogenin. Previously, it was demonstrated that, after conditionally depleting MuSCs in a Pax7-dependent manner, skeletal muscle did not or hardly regenerate after damage (20,21). In the absence of MuSCs, injured muscle became infiltrated with inflammatory cells and muscle tissue was replaced with fat and fibrotic tissue, further emphasizing the important role of MuSCs in mediating a healthy regenerative response. Earlier studies already found that skeletal muscle can still regenerate when MuSC numbers are at least 10% of the levels in healthy young animals (22), indicating a large “regenerative reserve” under healthy conditions.
A defect in MuSC activation in Pompe disease
Defects in MuSC activity have been reported for various neuromuscular diseases. These comprise effects on their proliferative potential (23-27), changes in MuSC numbers (25,28-31), and alterations in their potential to properly activate (32) or differentiate (33) (Table 1). Animal models for these and other muscle-degenerative diseases were also found to have defects in MuSC activation, proliferation, self-renewal, and differentiation potential (34-39) (Table 1). These effects of the failing MuSC population can be irreversible—as in certain dystrophies—or reversible as in Pompe disease.
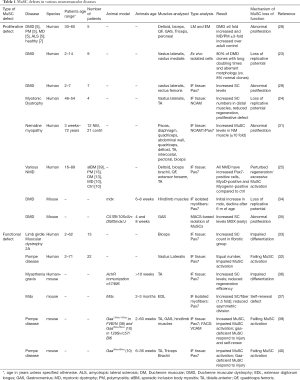
Full table
The current dogma for Duchenne muscular dystrophy (DMD) is that affected muscles undergo continuous rounds of de- and regeneration—as result of contractile myofiber damage—which is thought to eventually exhaust the MuSC pool [see for recent excellent reviews (41,42)]. This proliferative/exhaustive phenotype was observed in myogenic progenitors explanted from dystrophic muscle, with the defect more pronounced in cells isolated from older patients (23,43). Loss of proliferative potential has been attributed to attrition of telomere length (44), which was indeed demonstrated in samples from human DMD subjects (45). The loss of proliferative potential of MuSCs is not restricted to DMD, but also described for other muscle disorders, including myotonic dystrophy (24) or sporadic body inclusion myopathy (25) (Table 1). Another mechanism for loss of MuSC function is through premature differentiation of MuSCs, resulting in depletion of the self-renewing stem cell pool, such as has been reported for Emery-Dreifuss dystrophy (46,47). In addition, as it was reported for Myasthenia Gravis, the differentiation potential of MuSC progeny may be compromised, so that muscle regeneration cannot be successfully completed (36) (Table 1). In these examples, the MuSC defect is cell-intrinsic and is likely to be irreversible.
Our recent results show that the mechanism of MuSC inactivation in Pompe disease is different and, reversible. We previously described that MuSC numbers are stable in biopsies from Pompe disease patients across a large age-range (2 months–71.7 years) (32) and we and others demonstrated that in mouse models of Pompe disease MuSCs are not exhausted (39,40) (Table 1). In fact, MuSC numbers were increased in Gaa-ko-mice in two different backgrounds -fvb/n and the mixed c57/bl6 and 129sv background. Muscle biopsies from Pompe disease patients showed considerable muscle damage which is expected to elicit a robust regenerative response, but we found that MuSCs were not activated to repair the damage. Markers of active regeneration, which includes the expression of embryonic myosin heavy chain, were absent (32). Similarly, Gaa-deficient mice show muscle wasting, as reflected in decreasing muscle wet weight, and show limited MuSC activation and regeneration (39), reminiscent of the observations in human Pompe disease patients. Interestingly, Gaa-ko mice, despite completely lacking functional Gaa similarly as classic infantile patients, only develop a muscle phenotype from 15 weeks onwards. At this age, an initial mild regenerative response observed in younger animals, reflected by a gradual increase in myofiber central nucleation and low-level MuSC proliferation, is lost. It can be hypothesized that in young Gaa-ko mice the mild regenerative response delayed the onset of muscle wasting.
To determine if the observed regenerative defect could be explained by compromised MuSC function, we as well as Lagalice and colleagues, performed muscle-injury experiments. Both these studies showed that Gaa-deficient muscle regenerated efficiently after chemical injury (i.e., using BaCl2 or cardiotoxin), excluding a cell-intrinsic defect of Gaa-deficient MuSCs. Moreover, we also showed that Gaa-ko muscle regenerated completely after serial injury, indicating that the Gaa-deficient MuSCs are also capable of self-renewal (39). Our current work focuses on explaining this apparent paradox between MuSC inactivity during disease progression, while still capable of responding efficiently to exogenous damage. We hypothesize that the failing MuSC activation in Pompe disease may be explained by an inhibitory environment or by insufficient activation signals.
The role of autophagy in MuSC activation and muscle regeneration
In order to maintain the quiescent state, MuSCs require a basal level of autophagic activity (48,49). Disruption of autophagy during aging is shown to affect homeostasis of MuSCs and results in reduction of the pool of quiescent MuSCs in aging individuals (49). In fact, autophagic activity is one of the first processes to be upregulated during MuSC activation. While quiescent cells reside in a low metabolic and energetic state, autophagic activity is rapidly increased after damage to supply the increased demand for ATP, and reducing cofactors and amino acids needed to sustain proliferation (48). Tang and colleagues identified SIRT1 as critical regulator of autophagic flux in activating MuSCs. Loss of SIRT1 resulted in disturbed autophagic activity and delayed MuSC activation. Blocking autophagy disrupted MuSC activation (48,50). Paolini and colleagues found in mice that were genetically modified to express reduced levels of autophagic activity—by ablation of Atg16l1, a key component in autophagy—that the muscle regenerative response was delayed (51). The regenerating muscle of these mice contained increased levels of uncommitted MuSCs (Pax7+/MyoD−) and less activated/committed muscle progenitors that expressed MyoD. The authors concluded that failure to upregulate autophagic activity during the early stages of regeneration blocked full MuSC activation. While autophagic activity needs to be increased during the very early stages of MuSC activation, inhibition of autophagy in MuSC progeny is essential for completing regeneration (52). These studies indicate that autophagic activity is tightly controlled during different stages of regeneration, and may even be differentially regulated in different states of MuSC activation. The role of autophagy in MuSC activation and muscle regeneration under diseased conditions is still not entirely clear and warrant further study.
Disruption of autophagic activity contributes to neuromuscular disease
Inhibition as well as excessive activation of autophagy is found to induce muscle atrophy (53,54), suggesting a narrow window of autophagic activity during muscle homeostasis. Genetic inhibition of autophagy was sufficient to induce morphological features of myopathy, including myofiber vacuolization and decreasing fiber diameter (55,56). Disrupted autophagic flux has been observed in biopsies from DMD patients (57), in X-linked myopathy (58), Danon disease (59) and Pompe disease (60,61). Studies in animal models of these diseases support a prominent role of autophagic dysfunction in the disease development. Lysosomal dysfunction in Pompe disease results in accumulation of glycogen as well as autophagic debris, that eventually blocks autophagic flux (62) and disrupts endosomal trafficking. Loss of endosomal trafficking has dramatic effects on cellular health and might also adversely impact uptake of recombinant GAA that is used as ERT in Pompe disease. Defective autophagy also affects mitochondrial functioning, as damaged mitochondria are removed through autophagy—in a process called mitophagy (8). In Pompe disease reduced autophagic activity may contribute to the mitochondrial abnormalities that are frequently observed in muscle biopsies (11,63). Regulation of autophagy plays a prominent role in Pompe disease progression. More extensive reviews on the topic can be found elsewhere (64,65). It is hypothesized that normalization of autophagy restores the potential to regenerate muscle damage.
The role of the MuSC niche in muscle regeneration
Under homeostatic conditions the MuSC niche is defined by (I) the myofiber that conveys chemical, electrical, and mechanical signals, and (II) the basal lamina, which is largely composed of laminin, collagen, and proteoglycans (Figure 1). This polarized protective environment is completely remodeled after myofiber damage exposing MuSCs to several environmental factors that include chemical and electrical signals, auxiliary cells [reviewed in (66)] and matrix components. Interfering with the contact to either the myofiber and/or the basal lamina results in MuSC activation.
The damaged myofiber has an important role in engaging repair after injury. The MuSC-activating potential harnessed within myofibers was already demonstrated by Bischoff in 1986 (67). Bischoff described that exposure to saline extract from crushed myofibers pushed quiescent MuSCs into entering the cell cycle. It is now recognized that disruption of the sarcolemma induces the damaged myofiber to release signals that activate MuSCs, including nitric oxide (NO) (68) and matrix-remodeling proteins MMP-2, MMP-9 and MMP-10 (69,70). The MMP remodeling proteases release growth factors—such as HGF—that are embedded in the matrix. NO that is released by the damaged fiber or by recruited macrophages that also play an important role in liberating HGF (71). HGF binds to c-met—the receptor for HGF—that is expressed on quiescent MuSCs. MuSCs also express fibroblast growth factor receptors 1 and 4 (72), and respond to FGF2 that is expressed by the myofiber and accumulates under basal lamina during aging (73). In fact, purified extracts from aged myofibers compared to those from adult fibers were found to stimulate MuSC proliferation more potently (73), indicating that the changing myofiber niche directly impacts behavior of MuSCs.
The myofiber niche does not solely consist of chemical signals: resident and recruited cells play important roles in mediated muscle homeostasis and regeneration. Among the resident cells are fibro-adipogenic progenitors (FAPs), that are increasingly recognized for their crucial role in regenerative myogenesis (74,75). The role of the immune response after damage has been known for much longer (76). As mentioned above, damaged myofibers recruit immune cells, including neutrophils, eosinophils, and monocytes, that are crucial for proper muscle regeneration (77).
The niche is not only important during MuSC activation but also plays a pivotal role in maintaining quiescence under homeostatic conditions. For instance, heparan sulfate is the glycosaminoglycan component of many proteoglycans in the basal membrane and is dynamically regulated during MuSC activation (78). During aging 6-O sulfation specifically increased, augmenting FGF2-signaling, MuSC activation and age-dependent loss of MuSC quiescence (78). These data show that the composition of the niche can determine and mediate the MuSC response and that the niche composition is responsive to changes in the host.
The role of the niche in Pompe disease
Immunofluorescence analyses of patient biopsies as well as those from Gaa-deficient mice show that both the sarcolemma and basal lamina remain intact throughout the course of disease, although we did not analyze changes in niche composition. Also, we did not observe changes in recruitment of major immune populations, including macrophages and lymphocytes (unpublished observations). These findings suggest that myofiber rupturing and necrotic death do not seem to play a major role in Pompe disease muscle pathology. In light of the observed lack of MuSC activation, concomitant with the absence of cell-intrinsic defects (see above), it can be hypothesized that in Pompe disease the MuSC niche either fails to generate signals that activate MuSCs or that the niche actively suppresses MuSC activation. This may suggest that the niche could be a novel therapeutic target in Pompe disease.
The effect of targeting the niche is illustrated in merosin-deficiency or congenital muscular dystrophy 1A (CMD1A). CMD1A is caused by mutations in basal lamina protein LAMA2. It has been demonstrated that expression of integrin A7—that normally binds laminin—restores function and integrity of the basal lamina, and reduces muscle pathology in merosin-deficient mice (79).
Muscle-regenerative therapies for Pompe disease
Various experimental approaches have been developed to target specific regenerative defects in animal models of different muscle disorders. As was discussed above, the regenerative defect in Pompe disease is distinct from that observed in DMD, with insufficient MuSC activation vs. MuSC hyperactivation, respectively. The observed MuSC inactivity in Pompe disease suggests that focusing on safe and efficient methods to activate MuSCs may have the potential to attenuate muscle pathology in Pompe disease. As possible targets for regenerative therapy we will discuss approaches to activate MuSCs directly—providing signals that activate MuSCs—or indirectly, such as by restoring autophagic activity. Some of these approaches bypass the contribution of the endogenous niche, such as injecting growth factors, or involve the niche by inducing myofiber damage—as in the exercise approach. The aim of these strategies is not only to improve muscle morphology and function, but also to reduce the lysosomal damage and improve autophagic/endosomal activity. As a bystander effect, improving muscle morphology and function may positively affect the response to the current ERT therapy.
Activating MuSCs by exercise
Activation of MuSCs and increase in MuSC numbers after resistance exercise are well-documented (80-82), and occur even after a single bout of exercise (83-85). Several factors of the exercise program have been found to affect the MuSC response including mode of exercise—i.e., resistance vs. endurance—exercise intensity, duration of the program, age, and sex of the subjects [see also (86)]. Exercise programs are being used and appear well-tolerated for patients with various types of neuromuscular diseases, including DMD (87), Becker muscular dystrophy (BMD) (88), Myasthenia Gravis (89) and myotonic dystrophy (90). Beneficial effects of exercise have also been observed in patients with metabolic myopathies, including glycogen storage diseases (91,92). In our center we performed a 12-week exercise programs with Pompe disease patients consisting of 36 sessions of standardized aerobic, resistance, and core stability exercises (93,94). This study showed that exercise was well-tolerated by patients and improved endurance of patients’ muscles—as reflected by increased workload capacity, maximum oxygen uptake capacity and walking distance—and muscle function of hip flexors and shoulder abductors. It will be interesting to determine whether focused and safe exercise programs are capable of sufficiently activating MuSCs to restore the muscle regenerative response in Pompe disease patients.
Concerns exists regarding the safety and potential harmful effects of exercise in patients with increased sensitivity for contractile damage, such as in DMD. Exercise programs for such patients should probably minimize inclusion of lengthening eccentric contractions, which produces greater sarcolemmal disruptions. It should be noted that eccentric—but not concentric contractions—are associated with an efficient MuSC-mediated response (95). Such concerns are not as relevant for Pompe disease patients because of the lack of sarcolemmal fragility. Another concern is immobility of more advanced stage patients that may prevent participation in intensive exercise programs. Interestingly, pathways that were found to be affected by exercise, including AMPKA and PGC1a, can be activated by small molecule reagents—called exercise mimetics—and would offer the possibility of exercising less-mobile patients “pharmacologically”. Narkar and colleagues found that treating sedentary c57/bl6 mice with AICAR and PPARg agonist increased running endurance (96). AMPK signaling as potential target in modulating MuSC activation is supported by observations from Fu et al., who showed that inhibition of AMPK severely impairs satellite cell-mediated muscle regeneration (97).
Exercise programs for neuromuscular patients constitute a minimally invasive therapeutic modality that can be combined with the current state of care or with future therapeutic interventions. As mentioned above, for Pompe disease improving condition of affected muscles may attenuate disease progression and could even positively affect the response to ERT. Obviously, nutrition plays a role in optimizing the response to exercise, although that is beyond the scope of this review.
Activating MuSCs by soluble factors
MuSCs respond to soluble signals that are released into their direct environment following damage, including FGF2, HGF, and NO. Intraperitoneal injection of HGF was found to reverse experimental atrophy in mice and activate MuSCs, as determined by an increase in the fraction of MuSCs that expressed the cell cycle marker Ki67 (98). Interestingly, mTOR activity was reduced in muscle homogenates of treated animals, suggesting that changes in autophagic activity and protein synthesis may also be involved in the observed effect.
Activating MuSCs by targeting autophagy
Autophagic activity is a major determinant of MuSC activation and successful muscle regeneration, and is often dysregulated in neuromuscular patients, in particular in those with lysosomal storage disorders. The beneficial effects of exercise are also mediated through changes in autophagic activity in the skeletal muscle of mice (99) and humans (100). Exercise activates autophagy through increases in AMPK activity, which in turn inhibits mTORC1—an inhibitor of autophagy (101). The exercise mimetics that were discussed above activate AMPK signaling and function, at least partly, by increasing autophagic activity.
It is thought that glycogen traffics to the lysosome though autophagy (102) and as result of this process cellular debris is taken up as well. Disrupted autophagy as is observed in Pompe disease results in lysosomal accumulation of waste material that eventually terminates the autophagic flux. Based on this, it has been proposed that blocking autophagy would prevent the buildup of autophagic waste material and glycogen in Pompe disease and as such improve the condition of Gaa-deficient muscle. Indeed, autophagic buildup and glycogen accumulation were reduced in fast muscle from autophagy-deficient Gaa-KO mice, where Atg7—a critical member of the autophagy pathway—was depleted. The Gaa-/-/Atg7cKO mice actually appeared more healthy compared to, the “regular” Gaa-KO mice (103). Interestingly, the Gaa-/-/Atg7cKO mouse model also showed increased lysosomal glycogen clearing after ERT (103). A recent study showed different strategies targeting autophagy that resulted in removal of autophagic debris, reactivation of autophagic activity and restoration of the response to ERT in Gaa-KO mice (104), demonstrating the potential of autophagy as a therapeutic target. To determine the feasibility of autophagy as target for therapy, it will be informative to examine the energetic state and mTOR status of the stem cells in the affected muscles.
Increasing autophagy activity was also found to improve the muscle phenotype in the mouse model of DMD (105). In mdx mice autophagic activity is inhibited possibly as result of enhanced levels of oxidative stress, as determined using a Nox2-specific reactive oxygen species (ROS) biosensor. Oxidative stress was found to induce mTOR activity, which is a potent inhibitor of autophagy (105). Genetic inhibition of Nox2, which was found to mediate ROS development in mdx mice, reduced oxidative stress levels, reversed autophagic activity, and resulted in improvement of muscle morphological abnormalities and muscle function (105). A similar improvement of the condition of mdx mice was observed after a low-protein diet, which was thought to be mediated by attenuation of autophagic dysfunction (57). Together these and other studies suggested that autophagy may be a novel target in the treatment of DMD and Pompe disease and perhaps also for other neuromuscular diseases.
Cell-based therapies and ex vivo gene therapy
The strategies described above harness the regenerative potential of endogenous muscle stem cells. Such strategies are feasible for Pompe disease where the endogenous MuSC population is retained and functional. However, activating MuSCs without gene correction will not constitute a permanent cure.
Advances in gene therapy strategies show promise of achieving gene correction. Current strategies are mediated through adeno-associated virus (AAV) and predominantly target the liver. Gaa expressed in the liver is excreted into the circulation and is then taken up by affected muscle in a process called cross-correction (106). Intramuscular injection of AAV into the diaphragm has also been developed. In this regard, clinical trials using AAV-mediated gene therapy are currently ongoing and show a good safety profile, as was recently reported in children (107).
AAV-vectors carrying truncated versions of dystrophin—microdystrophin—were found to be safe and seemed effective in dystrophic mice and dogs (108) and are currently being evaluated in clinical trials. A disadvantage of AAV-vectors is that they do not integrate into the host genome and may be lost on the longer term. Other vector backbones, such as lentiviral vectors (109,110)—that integrate—are investigated for use in gene therapy strategies, including for Pompe disease and DMD. A more detailed discussion of these strategies is beyond the scope of this review and has been reviewed elsewhere (8,111).
Cell-based therapies offer the potential to replenish the MuSC pool with gene-corrected cells via ex vivo gene therapy. As during muscle regeneration muscle progenitors fuse to form new myofibers, donor cells share their genetic content with affected fibers. Furthermore, a major advantage of cell-based therapies is the opportunity for long-term improvement of affected muscles. In the 1990s major efforts were undertaken to explore myoblast transfer therapies, based on promising results obtained in the mdx mouse model (112,113). Unfortunately, for a number of reasons the human trials all turned out negative and little if any efficacy was shown (114). In more recent studies, transplantation of MuSCs was found to be much more effective compared to myoblast transfer (115-117), at least in mouse studies. This reignited interest in the development of a cell-based therapy for muscle-wasting disorders. The major drawback still is that MuSCs lose most of their regenerative potential when expanded ex vivo (118). The advent of induced pluripotent stem cells (IPSC) (119) opened novel opportunities of generating tissue-specific progenitor cells without the problems associated with expansion. We have recently described a method to generate skeletal muscle progenitors from Pompe disease patient fibroblasts that retain large expansion potential while maintaining the capacity to differentiate efficiently in vitro and engraft in vivo (120). The advantage of iPS cells as cell source is that these are amenable for ex vivo gene correction using CRISPR-Cas9, for example, to generate gene-corrected myogenic progenitors. Additionally, we used the IPS-derived muscle progenitor model to show the efficacy of an antisense oligonucleotide strategy to increase exon inclusion correcting a frequent splicing mutation found in the Pompe population (121,122). Splicing-targeted therapies for muscle disorders are a very fruitful and promising field and are recently reviewed elsewhere (123). The use of IPS-derived muscle progenitors as sources for cell-based therapies is also considered for other muscle-wasting disorders, including DMD, and has much potential. However, it is currently difficult to fully assess the clinical potential using small animal models, and further studies into the safety of the reprogramming and gene-editing strategies are required.
In vivo gene editing as a therapy
The development of current gene editing tools, in particular CRISPR-Cas9 technology, is rapidly moving forward the field of gene-correction strategies. Recently, this technology has been used to explore the efficacy of gene-editing the genetic defect in mdx (124-126). In these studies the CRISPR-Cas9 components were delivered using AAV vectors, and were delivered either directly intramuscularly or systemically. Long and colleagues showed by using this strategy in mdx mice that up to 25.5% of TA fibers expressed dystrophin after intramuscular delivery as judged by immunofluorescent analysis. Systemic—intraperitoneal administration in this study—was less efficient (125).
Tabebordbar and colleagues isolated MuSCs from the mdx/Pax7 zgreen+ compound reporter mouse, and demonstrated truncated DMD transcripts in MuSC-derived myotubes in vitro (126), suggesting that the MuSCs had indeed been targeted in vivo. Recently, early results—2 months follow-up—from an in vivo gene-editing study in a canine model of DMD using CRISPR/Cas9 technology were reported showing improved histology in four dogs. After systemic delivery dystrophin levels up to 90% of WT levels were found depending on the tissue analyzed (127).
A recent study in a DMD model in nonhuman primates also showed effectivity of in vivo gene editing (128). Despite these encouraging early results, caution is warranted. In vivo gene-editing is still associated with safety concerns (129) and is still rather inefficient. Further study is required for the development of safe and efficient gene-editing method for future clinical implementation.
Conclusions
In this review we focused on the mechanism of the observed failing regenerative response in Pompe disease and conclude that the failure to efficiently activate MuSCs contributes to ongoing muscle wasting. In the absence of muscle regeneration, muscle damage is not compensated by repair. To restore the regenerative balance, novel therapies may be directed to activate MuSCs directly—through direct administration of MuSC-activating agents—or indirectly, for instance by targeting autophagic activity. In particular, exercise therapy aimed to activate the inactive MuSC population is minimally invasive and has proven to be well-tolerated by patients. Based on the safety profile exercise therapy can be assumed to have clinical feasibility, although studies into their regenerative efficacy are warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van der Ploeg AT, Reuser AJ. Pompe’s disease. Lancet 2008;372:1342-53. [Crossref] [PubMed]
- Ebbink BJ, Aarsen FK, Van Gelder CM, et al. Cognitive outcome of patients with classic infantile Pompe disease receiving enzyme therapy. Neurology 2012;78:1512-8. [Crossref] [PubMed]
- Spiridigliozzi GA, Heller JH, Case LE, et al. Early cognitive development in children with infantile Pompe disease. Mol Genet Metab 2012;105:428-32. [Crossref] [PubMed]
- van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med 2010;362:1396-406. [Crossref] [PubMed]
- Van den Hout JM, Kamphoven JH, Winkel LP, et al. Long-Term Intravenous Treatment of Pompe Disease With Recombinant Human -Glucosidase From Milk. Pediatrics 2004;113:e448-57. [Crossref] [PubMed]
- Kuperus E, Kruijshaar ME, Wens SC, et al. Long-term benefit of enzyme replacement therapy in Pompe disease. Neurology 2017;89:2365-73. [Crossref] [PubMed]
- Bergsma AJ, In ‘t Groen SL, Verheijen FW, et al. From Cryptic Toward Canonical Pre-mRNA Splicing in Pompe Disease: a Pipeline for the Development of Antisense Oligonucleotides. Mol Ther Nucleic Acids 2016;5:e361.
- Kohler L, Puertollano R, Raben N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018;15:928-42. [Crossref] [PubMed]
- Bonne G, Rivier F, Hamroun D. The 2019 version of the gene table of neuromuscular disorders (nuclear genome). Neuromuscul Disord 2018;28:1031-63. [Crossref] [PubMed]
- Raben N, Nagaraju K, Lee E, et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998;273:19086-92. [Crossref] [PubMed]
- Thurberg BL, Lynch Maloney C, Vaccaro C, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest 2006;86:1208-20. [Crossref] [PubMed]
- Ebbink BJ, Poelman E, Plug I, et al. Cognitive decline in classic infantile Pompe disease: An underacknowledged challenge. Neurology 2016;86:1260-1. [Crossref] [PubMed]
- Seranova E, Connolly KJ, Zatyka M, et al. Dysregulation of autophagy as a common mechanism in lysosomal storage diseases. Essays Biochem 2017;61:733-49. [Crossref] [PubMed]
- Pichiecchio A, Uggetti C, Ravaglia S, et al. Muscle MRI in adult-onset acid maltase deficiency. Neuromuscul Disord 2004;14:51-5. [Crossref] [PubMed]
- Carlier RY, Laforet P, Wary C, et al. Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: Involvement patterns. Neuromuscul Disord 2011;21:791-9. [Crossref] [PubMed]
- van der Beek N a ME, de Vries JM, Hagemans ML, et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J Rare Dis 2012;7:88.
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 1961;9:493-5. [Crossref] [PubMed]
- Zammit PS, Golding JP, Nagata Y, et al. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol 2004;166:347-57. [Crossref] [PubMed]
- Shea KL, Xiang W, LaPorta VS, et al. Sprouty1 Regulates Reversible Quiescence of a Self-Renewing Adult Muscle Stem Cell Pool during Regeneration. Cell Stem Cell 2010;6:117-29. [Crossref] [PubMed]
- Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011;138:3639-46. [Crossref] [PubMed]
- von Maltzahn J, Jones AE, Parks RJ, et al. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A 2013;110:16474-9. [Crossref] [PubMed]
- Gayraud-Morel B, Chrétien F, Flamant P, et al. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol 2007;312:13-28. [Crossref] [PubMed]
- Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 1983;80:4856-60. [Crossref] [PubMed]
- Thornell LE, Lindstöm M, Renault V, et al. Satellite cell dysfunction contributes to the progressive muscle atrophy in myotonic dystrophy type 1. Neuropathol Appl Neurobiol 2009;35:603-13. [Crossref] [PubMed]
- Wanschitz JV, Dubourg O, Lacene E, et al. Expression of myogenic regulatory factors and myo-endothelial remodeling in sporadic inclusion body myositis. Neuromuscul Disord 2013;23:75-83. [Crossref] [PubMed]
- Renault V, Piron-Hamelin G, Forestier C, et al. Skeletal muscle regeneration and the mitotic clock. Exp Gerontol 2000;35:711-9. [Crossref] [PubMed]
- Decary S, Ben Hamida C, Mouly V, et al. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord 2000;10:113-20. [Crossref] [PubMed]
- Ishimoto S, Goto I, Ohta M, et al. A quantitative study of the muscle satellite cells in various neuromuscular disorders. J Neurol Sci 1983;62:303-14. [Crossref] [PubMed]
- Kottlors M, Kirschner J. Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res 2010;340:541-8. [Crossref] [PubMed]
- Wakayama Y. Electron microscopic study on the satellite cell in the muscle of Duchenne muscular dystrophy. J Neuropathol Exp Neurol 1976;35:532-40. [Crossref] [PubMed]
- Sanoudou D, Haslett JN, Kho AT, et al. Expression profiling reveals altered satellite cell numbers and glycolytic enzyme transcription in nemaline myopathy muscle. Proc Natl Acad Sci U S A 2003;100:4666-71. [Crossref] [PubMed]
- Schaaf GJ, van Gestel TJ, Brusse E, et al. Lack of robust satellite cell activation and muscle regeneration during the progression of Pompe disease. Acta Neuropathol Commun 2015;3:65. [Crossref] [PubMed]
- Rosales XQ, Malik V, Sneh A, et al. Impaired regeneration in LGMD2A supported by increased PAX7-positive satellite cell content and muscle-specific microrna dysregulation. Muscle Nerve 2013;47:731-9. [Crossref] [PubMed]
- Jiang C, Wen Y, Kuroda K, et al. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis Model Mech 2014;7:997-1004. [PubMed]
- Iannotti FA, Pagano E, Guardiola O, et al. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat Commun 2018;9:3950. [Crossref] [PubMed]
- Attia M, Maurer M, Robinet M, et al. Muscle satellite cells are functionally impaired in myasthenia gravis: consequences on muscle regeneration. Acta Neuropathol 2017;134:869-88. [Crossref] [PubMed]
- Dumont NA, Wang YX, von Maltzahn J, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 2015;21:1455-63. [Crossref] [PubMed]
- Bijvoet AG, Van De Kamp EH, Kroos MA, et al. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum Mol Genet 1998;7:53-62. [Crossref] [PubMed]
- Schaaf GJ, van Gestel TJM, in ‘t Groen SLM, et al. Satellite cells maintain regenerative capacity but fail to repair disease-associated muscle damage in mice with Pompe disease. Acta Neuropathol Commun 2018;6:119. [Crossref] [PubMed]
- Lagalice L, Pichon J, Gougeon E, et al. Satellite cells fail to contribute to muscle repair but are functional in Pompe disease (glycogenosis type II). Acta Neuropathol Commun 2018;6:116. [Crossref] [PubMed]
- Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol 2016;17:267-79. [Crossref] [PubMed]
- Feige P, Brun CE, Ritso M, et al. Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell 2018;23:653-64. [Crossref] [PubMed]
- Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: Implications for cell and gene therapy. Somat Cell Mol Genet 1990;16:557-65. [Crossref] [PubMed]
- Sacco A, Mourkioti F, Tran R, et al. Short telomeres and stem cell exhaustion model duchenne muscular dystrophy in mdx/mTR mice. Cell 2010;143:1059-71. [Crossref] [PubMed]
- Tichy ED, Sidibe DK, Tierney MT, et al. Single Stem Cell Imaging and Analysis Reveals Telomere Length Differences in Diseased Human and Mouse Skeletal Muscles. Stem Cell Reports 2017;9:1328-41. [Crossref] [PubMed]
- Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet 2006;7:940. [Crossref] [PubMed]
- Frock RL, Kudlow BA, Evans AM, et al. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev 2006;20:486-500. [Crossref] [PubMed]
- Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J 2014;33:2782-97. [Crossref] [PubMed]
- Sousa-Victor P, Gutarra S, García-Prat L, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014;506:316-21. [Crossref] [PubMed]
- Fiacco E, Castagnetti F, Bianconi V, et al. Autophagy regulates satellite cell ability to regenerate normal and dystrophic muscles. Cell Death Differ 2016;23:1839-49. [Crossref] [PubMed]
- Paolini A, Omairi S, Mitchell R, et al. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci Rep 2018;8:9062. [Crossref] [PubMed]
- Matsumoto A, Pasut A, Matsumoto M, et al. MTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017;541:228-32. [Crossref] [PubMed]
- Sandri M. Autophagy in skeletal muscle. FEBS Lett 2010;584:1411-6. [Crossref] [PubMed]
- Mammucari C, Milan G, Romanello V, et al. FoxO3 Controls Autophagy in Skeletal Muscle In Vivo. Cell Metab 2007;6:458-71. [Crossref] [PubMed]
- Masiero E, Agatea L, Mammucari C, et al. Autophagy Is Required to Maintain Muscle Mass. Cell Metab 2009;10:507-15. [Crossref] [PubMed]
- Masiero E, Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 2010;6:307-9. [Crossref] [PubMed]
- De Palma C, Morisi F, Cheli S, et al. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 2014;5:e1363. [Crossref] [PubMed]
- Dowling JJ, Moore SA, Kalimo H, et al. X-linked myopathy with excessive autophagy: a failure of self-eating. Acta Neuropathol 2015;129:383-90. [Crossref] [PubMed]
- Nascimbeni AC, Fanin M, Angelini C, et al. Autophagy dysregulation in Danon disease. Cell Death Dis 2017;8:e2565. [Crossref] [PubMed]
- Nascimbeni AC, Fanin M, Masiero E, et al. The role of autophagy in the pathogenesis of glycogen storage disease type II (GSDII). Cell Death Differ 2012;19:1698-708. [Crossref] [PubMed]
- Fukuda T, Ewan L, Bauer M, et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol 2006;59:700-8. [Crossref] [PubMed]
- Raben N, Hill V, Shea L, et al. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet 2008;17:3897-908. [Crossref] [PubMed]
- Schoser BGH, Müller-Höcker J, Horvath R, et al. Adult-onset glycogen storage disease type 2: Clinico-pathological phenotype revisited. Neuropathol Appl Neurobiol 2007;33:544-59. [PubMed]
- Rodríguez-Arribas M, Pedro JM, Gómez-Sánchez R, et al. Pompe Disease and Autophagy: Partners in Crime, or Cause and Consequence? Curr Med Chem 2016;23:2275-85. [Crossref] [PubMed]
- Lim JA, Zare H, Puertollano R, et al. Atg5flox-Derived Autophagy-Deficient Model of Pompe Disease: Does It Tell the Whole Story? Mol Ther Methods Clin Dev 2017;7:11-4. [Crossref] [PubMed]
- Mashinchian O, Pisconti A, Le Moal E, et al. The Muscle Stem Cell Niche in Health and Disease. Curr Top Dev Biol 2018;126:23-65. [Crossref] [PubMed]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol 1986;115:140-7. [Crossref] [PubMed]
- Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn 2007;236:240-50. [Crossref] [PubMed]
- Bobadilla M, Sáinz N, Rodriguez J, et al. MMP-10 is required for efficient muscle regeneration in mouse models of injury and muscular dystrophy. Stem Cells 2014;32:447-61. [Crossref] [PubMed]
- Kherif S, Lafuma C, Dehaupas M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 1999;205:158-70. [Crossref] [PubMed]
- Tatsumi R, Hattori A, Ikeuchi Y, et al. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 2002;13:2909-18. [Crossref] [PubMed]
- Pawlikowski B, Vogler TO, Gadek K, et al. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev Dyn 2017;246:359-67. [Crossref] [PubMed]
- Chakkalakal JV, Jones KM, Basson MA, et al. The aged niche disrupts muscle stem cell quiescence. Nature 2012;490:355-60. [Crossref] [PubMed]
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010;12:153-63. [Crossref] [PubMed]
- Mueller AA, Van Velthoven CT, Fukumoto KD, et al. Intronic polyadenylation of PDGFRα in resident stem cells attenuates muscle fibrosis. Nature 2016;540:276-9. [Crossref] [PubMed]
- Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 2017;17:165-78. [Crossref] [PubMed]
- Heredia JE, Mukundan L, Chen FM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013;153:376-88. [Crossref] [PubMed]
- Ghadiali RS, Guimond SE, Turnbull JE, et al. Dynamic changes in heparan sulfate during muscle differentiation and ageing regulate myoblast cell fate and FGF2 signalling. Matrix Biol 2017;59:54-68. [Crossref] [PubMed]
- Doe JA, Wuebbles RD, Allred ET, et al. Transgenic overexpression of the α7 integrin reduces muscle pathology and improves viability in the dy(W) mouse model of merosin-deficient congenital muscular dystrophy type 1A. J Cell Sci 2011;124:2287-97. [Crossref] [PubMed]
- Charifi N, Kadi F, Féasson L, et al. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 2003;28:87-92. [Crossref] [PubMed]
- Kadi F, Eriksson A, Holmner S, et al. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol 1999;111:189-95. [Crossref] [PubMed]
- Verdijk LB, Snijders T, Drost M, et al. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2014;36:545-7. [Crossref] [PubMed]
- Snijders T, Verdijk LB, Beelen M, et al. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp Physiol 2012;97:762-73. [Crossref] [PubMed]
- Bellamy LM, Joanisse S, Grubb A, et al. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 2014;9:e109739. [Crossref] [PubMed]
- Dreyer HC, Blanco CE, Sattler FR, et al. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 2006;33:242-53. [Crossref] [PubMed]
- Bazgir B, Fathi R, Rezazadeh Valojerdi M, et al. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J 2017;18:473-84. [PubMed]
- Jansen M, van Alfen N, Geurts AC, et al. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial "no use is disuse". Neurorehabil Neural Repair 2013;27:816-27. [Crossref] [PubMed]
- Sveen ML, Jeppesen TD, Hauerslev S, et al. Endurance training improves fitness and strength in patients with Becker muscular dystrophy. Brain 2008;131:2824-31. [Crossref] [PubMed]
- Lohi EL, Lindberg C, Andersen O. Physical training effects in myasthenia gravis. Arch Phys Med Rehabil 1993;74:1178-80. [PubMed]
- Tollbäck A, Eriksson S, Wredenberg A, et al. Effects of high resistance training in patients with myotonic dystrophy. Scand J Rehabil Med 1999;31:9-16. [Crossref] [PubMed]
- Vissing J. Exercise training in metabolic myopathies. Rev Neurol (Paris) 2016;172:559-65. [Crossref] [PubMed]
- Preisler N, Haller RG, Vissing J. Exercise in muscle glycogen storage diseases. J Inherit Metab Dis 2015;38:551-63. [Crossref] [PubMed]
- Favejee MM, van den Berg LE, Kruijshaar ME, et al. Exercise training in adults with Pompe disease: the effects on pain, fatigue, and functioning. Arch Phys Med Rehabil 2015;96:817-22. [Crossref] [PubMed]
- van den Berg LE, Favejee MM, Wens SC, et al. Safety and efficacy of exercise training in adults with Pompe disease: evalution of endurance, muscle strength and core stability before and after a 12 week training program. Orphanet J Rare Dis 2015;10:87. [Crossref] [PubMed]
- Hyldahl RD, Olson T, Welling T, et al. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front Physiol 2014;5:485. [Crossref] [PubMed]
- Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008;134:405-15. [Crossref] [PubMed]
- Fu X, Zhu M, Zhang S, et al. Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK. Diabetes 2016;65:188-200. [PubMed]
- Hauerslev S, Vissing J, Krag TO. Muscle atrophy reversed by growth factor activation of satellite cells in a mouse muscle atrophy model. PLoS One 2014;9:e100594. [Crossref] [PubMed]
- Lenhare L, Crisol BM, Silva VRR, et al. Physical exercise increases Sestrin 2 protein levels and induces autophagy in the skeletal muscle of old mice. Exp Gerontol 2017;97:17-21. [Crossref] [PubMed]
- Schwalm C, Jamart C, Benoit N, et al. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J 2015;29:3515-26. [Crossref] [PubMed]
- Hardie DG. AMPK and autophagy get connected. EMBO J 2011;30:634-5. [Crossref] [PubMed]
- Kotoulas OB, Kalamidas SA, Kondomerkos DJ. Glycogen autophagy in glucose homeostasis. Pathol Res Pract 2006;202:631-8. [Crossref] [PubMed]
- Raben N, Schreiner C, Baum R, et al. Suppression of autophagy permits successful enzyme replacement therapy in a lysosomal storage disorder--murine Pompe disease. Autophagy 2010;6:1078-89. [Crossref] [PubMed]
- Lim JA, Sun B, Puertollano R, et al. Therapeutic Benefit of Autophagy Modulation in Pompe Disease. Mol Ther 2018;26:1783-96. [Crossref] [PubMed]
- Pal R, Palmieri M, Loehr JA, et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun 2014;5:4425. [Crossref] [PubMed]
- Puzzo F, Colella P, Biferi MG, et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase. Sci Transl Med 2017. [Crossref] [PubMed]
- Corti M, Liberati C, Smith BK, et al. Safety of Intradiaphragmatic Delivery of Adeno-Associated Virus-Mediated Alpha-Glucosidase (rAAV1-CMV-hGAA) Gene Therapy in Children Affected by Pompe Disease. Hum Gene Ther Clin Dev 2017;28:208-18. [Crossref] [PubMed]
- Le Guiner C, Servais L, Montus M, et al. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat Commun 2017;8:16105. [Crossref] [PubMed]
- Counsell JR, Asgarian Z, Meng J, et al. Lentiviral vectors can be used for full-length dystrophin gene therapy. Sci Rep 2017;7:79. [Crossref] [PubMed]
- Van Til NP, Stok M, Aerts Kaya FS, et al. Lentiviral gene therapy of murine hematopoietic stem cells ameliorates the Pompe disease phenotype. Blood 2010;115:5329-37. [Crossref] [PubMed]
- Chamberlain JR, Chamberlain JS. Progress toward Gene Therapy for Duchenne Muscular Dystrophy. Mol Ther 2017;25:1125-31. [Crossref] [PubMed]
- Partridge TA, Morgan JE, Coulton GR, et al. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 1989;337:176-9. [Crossref] [PubMed]
- Partridge TA, Grounds M, Sloper JC. Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature 1978;273:306-8. [Crossref] [PubMed]
- Briggs D, Morgan JE. Recent progress in satellite cell/myoblast engraftment -- relevance for therapy. FEBS J 2013;280:4281-93. [Crossref] [PubMed]
- Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005;122:289-301. [Crossref] [PubMed]
- Sacco A, Doyonnas R, Kraft P, et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008;456:502-6. [Crossref] [PubMed]
- Cerletti M, Jurga S, Witczak CA, et al. Highly Efficient, Functional Engraftment of Skeletal Muscle Stem Cells in Dystrophic Muscles. Cell 2008;134:37-47. [Crossref] [PubMed]
- Schaaf GJ. Ex-vivo Expansion of Muscle-Regenerative Cells for the Treatment of Muscle Disorders. J Stem Cell Res Ther 2012;01:003.
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- van der Wal E, Herrero-Hernandez P, Wan R, et al. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Reports 2018;10:1975-90. [Crossref] [PubMed]
- van der Wal E, Bergsma AJ, van Gestel TJ, et al. GAA Deficiency in Pompe Disease Is Alleviated by Exon Inclusion in iPSC-Derived Skeletal Muscle Cells. Mol Ther Nucleic Acids 2017;7:101-15. [Crossref] [PubMed]
- van der Wal E, Bergsma AJ, Pijnenburg JM, et al. Antisense Oligonucleotides Promote Exon Inclusion and Correct the Common c.-32-13T>G GAA Splicing Variant in Pompe Disease. Mol Ther Nucleic Acids 2017;7:90-100. [Crossref] [PubMed]
- Bergsma AJ, van der Wal E, Broeders M, van der Ploeg AT, Pim Pijnappel WWM. Alternative Splicing in Genetic Diseases: Improved Diagnosis and Novel Treatment Options. Int Rev Cell Mol Biol 2018;335:85-141. [Crossref] [PubMed]
- Nelson CE, Hakim CH, Ousterout DG, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016;351:403-7. [Crossref] [PubMed]
- Long C, Amoasii L, Mireault AA, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 2016;351:400-3. [Crossref] [PubMed]
- Tabebordbar M, Zhu K, Cheng JK, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016;351:407-11. [Crossref] [PubMed]
- Amoasii L, Hildyard JC, Li H, et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 2018;362:86-91. [Crossref] [PubMed]
- Wang S, Ren S, Bai R, et al. No off-target mutations in functional genome regions of a CRISPR/Cas9-generated monkey model of muscular dystrophy. J Biol Chem 2018;293:11654-8. [Crossref] [PubMed]
- Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol 2018;36:765-71. [Crossref] [PubMed]


