
Improving patient selection for adjuvant therapy in high-risk renal cell carcinoma
From 2007 to 2011, the adjuvant sunitinib in high-risk renal cell carcinoma (RCC) after nephrectomy (S-TRAC) investigators conducted a phase 3, randomized, placebo-controlled trial with primary results published in 2016 (1). Patients with completely resected, high-risk RCC defined based on pathologic stage received either adjuvant sunitinib or placebo for a maximum of 1 year. Disease-free survival (DFS) was 6.8 years [95% confidence interval (CI): 5.8 to not reached] in patients receiving adjuvant sunitinib compared to 5.6 years (95% CI: 3.8–6.6) in patients receiving placebo (hazard ratio 0.76; 95% CI: 0.59–0.98; P=0.03). While this trial met its primary end point for DFS and ultimately led to FDA approval for sunitinib in the adjuvant setting, a benefit in overall survival (OS) was not seen. Similarly, subsequent trials have been unable to demonstrate an OS benefit for adjuvant systemic therapy following surgery for high risk RCC (2-5). A recent meta-analysis of adjuvant targeted therapies (including data from S-TRAC) found no DFS, cancer-specific survival (CSS), or OS benefits in patients receiving adjuvant treatment (6). Since up to 40% of patients with stage III/IV non-metastatic RCC are not cured with surgery alone (7-9), systemic therapies that can improve CSS and OS are eagerly sought.
Despite mostly negative results from large, randomized, phase III trials, secondary and subgroup analyses of select patients demonstrate a faint signal that may be promising (3-5). Similar to the findings in S-TRAC, improved DFS was observed for a subset of patients in the axitinib versus placebo as an adjuvant treatment of RCC (ATLAS) trial (4). In this trial, patients were randomized to receive either axitinib or placebo. Although the primary endpoint of DFS as determined by an independent review committee was not met, a significant DFS benefit was found in the highest-risk subgroup of patients. These patients were defined as having pT3 with Fuhrman Grade ≥3 or pT4 and/or N+, any T, any Fuhrman Grade disease, and a pre-specified P value ≤0.1352 was used to define statistical significance. In this subgroup of patients, investigator review found a DFS risk reduction of 36% (HR 0.641; 95% CI: 0.468–0.879, P=0.0051), while an independent review committee found a DFS risk reduction of 27% (HR 0.735; 95% CI: 0.525–1.028, P=0.0704). Taken together, these results suggest the possibility that patient selection rather than the therapeutic intervention itself may drive improved outcomes.
Biomarker-guided therapies have been used to target malignancies in other organ systems. Breast cancers now ubiquitously undergo molecular testing, the results of which determine the need for adjuvant therapy and have demonstrated improved survival outcomes in the adjuvant setting (10). Estrogen receptor positivity has been utilized since the 1970s for prognosticative and therapeutic input, with the selective estrogen receptor modulator tamoxifen demonstrating mortality benefits at both short-term and long-term follow-up (11). Detection of human epidermal growth factor receptor 2 (HER2) overexpression has also yielded improved progression and survival outcomes in patients treated with HER2-targeted therapies, which include trastuzumab, lapatinib, pertuzumab, or ado-trastuzumab emtansine (12).
The NCCN guidelines for colon cancer include tumor genotyping for KRAS, NRAS, BRAF, and microsatellite instability (MSI) or mismatch repair (MMR) (13). Mutations in KRAS and NRAS are predictive of a lack of response to treatment with cetuximab and panitumumab, allowing for these drugs (and their associated side effects) to be avoided in mutation-positive patients (14-16). Similarly, patients found to have the BRAF-V600E mutation are unlikely to respond to panitumumab or cetuximab, and alternative therapy should therefore be sought in patients with this mutation (17-19). Finally, patients with a deficient MMR phenotype have been shown to have a favorable prognosis following adjuvant FOLFOX therapy (20). Thus, breast and colon cancer provide a model by which patient selection for adjuvant therapies in RCC can be shaped.
The first study to demonstrate that biomarkers may help improve patient selection for adjuvant therapies in RCC was the adjuvant weekly girentuximab following nephrectomy for high-risk RCC (ARISER) trial (5). In ARISER, patients with high-risk RCC were randomized to receive either adjuvant girentuximab or placebo. Girentuximab is a monoclonal antibody against carbonic anhydrase IX (CAIX), a cell surface glycoprotein that is expressed in 95% of clear cell RCC tumors, but is not expressed by non-cancerous renal tissue (5,21). While no benefits in DFS (HR 0.97; 95% CI: 0.79–1.18) or OS (HR 0.99; 95% CI: 0.74–1.32) were observed in the intervention arm, a subgroup analysis was performed to explore the relationship between tumor CAIX expression and patient response to treatment. In this analysis, patients whose tumors demonstrated higher expression of CAIX experienced an improvement in DFS that approached statistical significance (HR 0.75; 95% CI: 0.55–1.04; P=0.08). These results blended support to the concept that certain biomarkers can predict improved response to treatment in RCC and may be a more refined instrument with which to select patients for adjuvant therapies compared to pathologic stage alone.
Increased expression of vascular endothelial growth factor (VEGF) is a well-established driver of tumorigenesis in RCC (22-25). VEGF binds the transmembrane receptors VEGFR-1 and VEGFR-2, through which it can affect a number of cell signaling pathways promoting tumor angiogenesis and survival (26). Targeted therapies for RCC work by blocking VEGF receptors, thereby potentiating the effects of VEGF overexpression in RCC. In patients with metastatic RCC, toxicity/need for dose-reduction (27,28), OS (29-32), progression-free survival (27,29-33), and response to treatment (29,30,33) have all been associated with various single nucleotide polymorphisms (SNPs), which are variations in single base pairs in the genome. These positive results evaluating SNP-guided prediction of treatment efficacy in metastatic RCC form the basis for exploring their use to more appropriately select patients for adjuvant therapy.
George and colleagues evaluated ten SNPs in genes associated with RCC tumorigenesis (34). Three SNPs were associated with statistically significant improvements in DFS favoring sunitinib over placebo: the C/C genotype for VEGFR1 rs9554320 (HR 0.44; 95% CI: 0.21–0.91; P=0.023), the T/T genotype for VEGFR2 rs2071559 (HR 0.46; 95% CI: 0.23–0.90; P=0.020), and the T/T genotype for endothelial nitric oxide synthase (eNOS) rs2070744 (HR 0.53; 95% CI: 0.30–0.94; P=0.028) (Table 1) (34). A fourth, the A/A genotype for VEGFR1 rs9582036, also trended toward longer DFS in the group that received sunitinib (HR 0.56; 95% CI: 0.30–1.02; P=0.054). Notably, assessment of OS among SNPs in the sunitinib versus placebo groups showed no significant differences; however, data were not fully mature when analyzed. A significantly longer OS was found in a combined analysis of the intervention and placebo groups when there was no insertion of CCDC26 rs60315789 (“–/–”) compared to the heterozygous (“–/TAT”) genotype, indicating its potential value as a post-surgical prognostic biomarker.
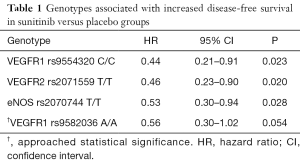
Full table
These findings should be considered hypothesis-generating for several reasons. Of 615 patients randomized in S-TRAC, fewer than half (286/615, 47%) had blood samples drawn for genotyping (34). Additionally, patients who were genotyped tended to be white (90.2% vs. 78.4%, P<0.001), older (age ≥65: 31.8% vs. 20.4%, P=0.002), and were considered to be higher-risk (University of California Los Angeles Integrated Staging System group T3 low: 30.1% vs. 42.9%, P=0.009) when compared to those who were not genotyped (34). Any of these between-group differences could have accounted for observed differences in outcomes. Therefore, these conclusions should be considered associative rather than causative. Nonetheless, it is plausible that the longer DFS associated with certain subgroups of patients who received sunitinib may have been due to DNA-level differences compared to non-responders. These findings require confirmation in prospective, randomized, placebo-controlled trials.
Beyond the intuitive benefit of having the ability to predict who will respond more favorably to adjuvant treatment, accurate prediction of who will not benefit from treatment is critically important. Sunitinib has a large adverse event profile that affects a significant number of patients treated (Table 2). In the S-TRAC trial, 48.4% of patients on sunitinib experienced grade 3 toxicities compared to only 15.8% in the placebo group; 12.1% of patients in the sunitinib group experienced grade 4 toxicities compared to only 3.6% in the placebo group (1). Should data confirming the ability to predict responders from non-responders be solidified in future prospective trials, non-responders could be preferentially offered alternative therapies.
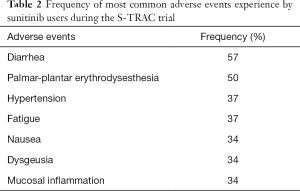
Full table
To date, adjuvant trials in RCC have been disappointing. However, it is possible that pathologic stage is simply too blunt an instrument to accurately select patients for adjuvant therapies. Therapy for any disease is not truly optimized until patients can be reliably stratified based on who is most likely to benefit from, suffer from, or be unaffected by any given intervention. While prospective studies are clearly warranted, George and colleagues provide provocative data suggesting that SNP analysis may help us better define the population most likely to benefit from adjuvant treatment in RCC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375:2246-54. [Crossref] [PubMed]
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008-16. [Crossref] [PubMed]
- Motzer RJ, Haas NB, Donskov F, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol 2017;35:3916-23. [Crossref] [PubMed]
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol 2018;29:2371-8. [Crossref] [PubMed]
- Chamie K, Donin NM, Klopfer P, et al. Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma: The ARISER Randomized Clinical Trial. JAMA Oncol 2017;3:913-20. [Crossref] [PubMed]
- Sonbol MB, Firwana B, Hilal T, et al. Adjuvant Antiangiogenic Agents in Post-nephrectomy Renal Cell Carcinoma: A Systematic Review and Meta-analysis. Eur Urol Oncol 2018;1:101-8. [Crossref] [PubMed]
- Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 2001;19:1649-57. [Crossref] [PubMed]
- Lam JS, Shvarts O, Leppert JT, et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol 2005;174:466-72; discussion 472; quiz 801. [Crossref] [PubMed]
- Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol 2004;22:3316-22. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 1.2019) 2019 [April 15, 2019]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Schink JC, Trosman JR, Weldon CB, et al. Biomarker testing for breast, lung, and gastroesophageal cancers at NCI designated cancer centers. J Natl Cancer Inst 2014. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (Version 1.2019) 2019 [April 15, 2019]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 2008;26:374-9. [Crossref] [PubMed]
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34. [Crossref] [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [Crossref] [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [Crossref] [PubMed]
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75. [Crossref] [PubMed]
- Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 2015;51:587-94. [Crossref] [PubMed]
- Zaanan A, Shi Q, Taieb J, et al. Role of Deficient DNA Mismatch Repair Status in Patients With Stage III Colon Cancer Treated With FOLFOX Adjuvant Chemotherapy: A Pooled Analysis From 2 Randomized Clinical Trials. JAMA Oncol 2018;4:379-83. [Crossref] [PubMed]
- Oosterwijk E, Bander NH, Divgi CR, et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol 1993;11:738-50. [Crossref] [PubMed]
- George DJ, Kaelin WG Jr. The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N Engl J Med 2003;349:419-21. [Crossref] [PubMed]
- Igarashi H, Esumi M, Ishida H, et al. Vascular endothelial growth factor overexpression is correlated with von Hippel-Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer 2002;95:47-53. [Crossref] [PubMed]
- Nicol D, Hii SI, Walsh M, et al. Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol 1997;157:1482-6. [Crossref] [PubMed]
- Takahashi A, Sasaki H, Kim SJ, et al. Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 1994;54:4233-7. [PubMed]
- Suarez C, Morales R, Munoz E, et al. Molecular basis for the treatment of renal cell carcinoma. Clin Transl Oncol 2010;12:15-21. [Crossref] [PubMed]
- Garcia-Donas J, Esteban E, Leandro-Garcia LJ, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol 2011;12:1143-50. [Crossref] [PubMed]
- Kim HR, Park HS, Kwon WS, et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol 2013;72:825-35. [Crossref] [PubMed]
- Beuselinck B, Karadimou A, Lambrechts D, et al. VEGFR1 single nucleotide polymorphisms associated with outcome in patients with metastatic renal cell carcinoma treated with sunitinib - a multicentric retrospective analysis. Acta Oncol 2014;53:103-12. [Crossref] [PubMed]
- Beuselinck B, Jean-Baptiste J, Schoffski P, et al. Validation of VEGFR1 rs9582036 as predictive biomarker in metastatic clear-cell renal cell carcinoma patients treated with sunitinib. BJU Int 2016;118:890-901. [Crossref] [PubMed]
- Dornbusch J, Walter M, Gottschalk A, et al. Evaluation of polymorphisms in angiogenesis-related genes as predictive and prognostic markers for sunitinib-treated metastatic renal cell carcinoma patients. J Cancer Res Clin Oncol 2016;142:1171-82. [Crossref] [PubMed]
- Scartozzi M, Bianconi M, Faloppi L, et al. VEGF and VEGFR polymorphisms affect clinical outcome in advanced renal cell carcinoma patients receiving first-line sunitinib. Br J Cancer 2013;108:1126-32. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Hudes GR, et al. Investigation of novel circulating proteins, germ line single-nucleotide polymorphisms, and molecular tumor markers as potential efficacy biomarkers of first-line sunitinib therapy for advanced renal cell carcinoma. Cancer Chemother Pharmacol 2014;74:739-50. [Crossref] [PubMed]
- George DJ, Martini JF, Staehler M, et al. Phase III Trial of Adjuvant Sunitinib in Patients with High-Risk Renal Cell Carcinoma: Exploratory Pharmacogenomic Analysis. Clin Cancer Res 2019;25:1165-73. [Crossref] [PubMed]


