
Seronegative atypical anti-glomerular basement membrane crescentic glomerulonephritis
Introduction
Anti-glomerular basement membrane (anti-GBM) disease in the kidney is characterized by linear deposition of immunoglobulin G (IgG) along the GBMs, directed against the non-collagenous domain of the alpha-3 chain (α3NC1) of type IV collagen (1,2). Seronegative anti-GBM disease is very rare, occurring in 2% to 3% of cases (3). We report a patient who presented with acute kidney injury and whose renal biopsy revealed pathological features of anti-GBM crescentic glomerulonephritis (GN) with serology negative for anti-GBM antibody.
Case presentation
A 46-year-old female was admitted to the hospital with a chief complaint of episodic hematuria for almost a month prior to admission. The patient was treated with ciprofloxacin for a presumed urinary tract infection but continued to have hematuria. The patient also complained of fatigue, moderate shortness of breath, and bilateral lower extremity swelling for few days. Her past medical history was significant for hypertension, hyperlipidemia and chronic obstructive pulmonary disease. The patient had a 60 pack-year smoking history and occasional alcohol intake. Her physical examination was significant for an elevated blood pressure of 171/92 mmHg, moderate respiratory distress, crepitations in both lung fields, and bilateral lower extremity edema. Laboratory studies revealed anemia with a hemoglobin 8.7 g/dL, a serum bicarbonate of 18 mmol/L, a serum blood urea nitrogen (BUN) of 85 mg/dL, and a serum creatinine of 10.81 mg/dL. Visual inspection of the urine revealed red color and haziness. Urinalysis showed 500 mg/dL protein, 150 mg/dL glucose, and 4+ blood. Urine microscopy further revealed dysmorphic red blood cells with >185 red cells/high powered field. Serological workup showed a non-reactive hepatitis panel, normal serum complement levels (C3: 132.7 mg/dL, C4: 35.7 mg/dL) and negative ANA, ANCA IgG, and anti-GBM IgG antibodies by indirect immunofluorescence (IIF) assay. Urine spot protein creatinine ratio was 17 mg/gm.
Her chest X-ray revealed trace bilateral pleural effusions. A computed tomography (CT) of chest without contrast revealed trace bilateral pleural effusion, minimal bibasilar air space disease suggestive but not diagnostic of atelectasis, trace pericardial effusion. A CT of the abdomen and pelvis without contrast revealed poor definition of the pelvicalyceal collecting systems of unclear etiology without evidence of hydronephrosis, renal calculi, or perinephric edema. Renal ultrasound showed a right kidney measuring 11.2 centimeters and a left kidney measuring 12.7 centimeters, with increased renal cortical echogenicity and the urinary bladder wall measuring up to 5 mm in thickness. Echocardiogram revealed a left ventricular ejection fraction of 44%, moderately decreased global left ventricular systolic dysfunction, and grade 2 diastolic dysfunction. Nephrology and Urology services were consulted for acute renal failure and hematuria from the emergency department. The patient was initiated on emergent hemodialysis for fluid overload and acute kidney injury and underwent cystoscopy which showed catheter related changes involving the bladder mucosa without any tumors and hence no bladder biopsy was obtained. However, light bloody effluxes from both the ureteral orifices were noted, suspicious for intrinsic renal disease contributing to hematuria.
A renal biopsy was performed. Light microscopy revealed diffuse glomerular involvement (greater than 90%) by crescents displaying temporal heterogeneity. Thirty-five glomeruli were identified, none of which were globally sclerotic. Nineteen glomeruli displayed segmental to global involvement by cellular crescents (Figure 1A), many of which were also associated with fibrinoid necrosis and GBM rupture. Four glomeruli contained global subacute (fibrocellular) crescents (Figure 1B), and two glomeruli contained remote/fibrous crescents (Figure 1C). The underlying glomeruli were histologically unremarkable without any evidence of underlying proliferative features. Immunofluorescence revealed strong, diffuse, linear staining of glomerular capillary basement membranes by IgG (Figure 1D).
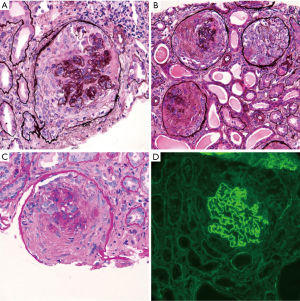
IIF staining for IgG subtypes revealed dominant staining for IgG4 (3+), with slightly lesser intensity staining with IgG1 (2–3+). Staining for IgG2 was weak (1+), and staining for IgG3 was negative. There was no other significant glomerular staining for immune reactants including complement. Electron microscopy performed on two glomeruli without crescentic involvement revealed GBMs with normal trilaminar structure and thickness. No ultrastructural abnormalities or ruptures were identified. Repeat anti-GBM antibody, IgG by multiplex bead test was negative. Although the patient was treated with pulse dose steroids and cyclophosphamide, the patient ultimately developed infectious complications from immunosuppression, and treatment was terminated. She remained dialysis dependent for 6 months and unfortunately died from cardiovascular complications.
Discussion
Anti-GBM disease is an autoimmune, self-limiting disease affecting mainly the capillaries of the kidneys and lungs (4). It was first described in 1967 by Lerner et al. (5). It is very rare with an incidence of one per million with a bimodal age distribution mainly in the third and sixth decades with a greater male predilection (6). Anti-GBM disease associated with pulmonary hemorrhage is referred to as Goodpasture’s syndrome.
All epithelial cell basement membranes have collagen as the primary structural protein. Specifically, a major structural component of human basement membranes is type IV collagen, which is composed of six distinct alpha chains. The alpha 3 chain is limited to specific organs, one of which is the kidney (7). Also known as the Goodpasture antigen, the non-collagenous domain of the alpha-3 chain usually escapes immune surveillance by being hidden through interactions with other non-collagenous domains (7). The major antibody implicated in the pathogenesis of anti-GBM disease is the IgG subtype, directed against major epitopes in the non-collagenous domain of type IV collagen (1,2). Autoantibodies directed against α5NC1, α4NC1, and linear type IV collagen epitopes have been described in addition to classic antibody. Other immunoglobulin subclasses have rarely been implicated (8,9).
A very high index of clinical suspicion needs to be maintained in diagnosing anti-GBM disease. Serologic detection of antibodies aids in prompt diagnosis which is confirmed with renal biopsy. The histological hallmark for diagnosis for anti-GBM disease is the linear deposition of IgG along the GBMs detected by immunofluorescence staining. Diagnostic dilemmas arise when commercially available serologic assays fail to test positive, thus highlighting the crucial role of renal biopsy for diagnosis. However, when anti-GBM disease presents atypically and with negative serologies, it may result in delayed diagnosis and treatment, which can be detrimental.
Multiple serological assays are available for detecting anti-GBM antibodies, including IIF, radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), chemiluminescence and Western blot (WB). The most commonly used serological assays are ELISA-based, which have a high sensitivity and specificity. WB and chemiluminescence have the highest sensitivity while direct immunofluorescence has the lowest sensitivity (3,10-13). Assays using extracted antigen have a lower specificity compared to human recombinant GBM antigens (11). If conventional testing as described above fails to detect anti-GBM antibodies, IgG4 mediated anti-GBM disease should be suspected (8).
There have been various epitopes described in the pathogenesis of anti-GBM disease. The most commonly implicated is the Ea epitope (14). Hellmark et al. described a series of crucial amino acid residues labeled as S2 (15), similarly Netzer et al. labelled as Ea and Eb respectively, all of which could elicit a pathogenic autoantibody response (16).
Table 1 lists possible mechanisms for false negative results (17).
In case series described by Nasr et al. he presented 20 patients with atypical anti-GBM nephritis characterized with undetectable circulating anti-GBM antibodies with no pulmonary involvement and indolent renal course. Pulmonary involvement occurs in typical anti-GBM disease in approximately 1/3 to 2/3 of patients. Inflammatory response incited by binding of autoimmune antibodies to GBM results in complement activation, inflammatory cell recruitment and GBM destruction leading to crescent formation and rapidly progressive GN. Atypical anti-GBM nephritis leads to less complement activation as described in this series. Atypical anti-GBM is characterized by mild renal failure, high grade proteinuria, and hematuria. In this case series light microscopic features varied revealing endocapillary proliferative GN, membranoproliferative GN, mesangial proliferative GN and focal segmental glomerulosclerosis with mesangial hypercellularity along with microangiopathy in 40% of these patients. Crescents were focal in 40% of the patients and absent in 60% of the patients (18).
In case series described by Liang et al. antibody-negative anti-GBM disease in 19 patients, dominant immunoglobulin subtype on Immunofluorescence was IgG4 or IgG1 (19). The finding of dominant immunofluorescence staining for the IgG4 subclass in this case is also atypical, as at least one previous study has reported IgG3 as the most frequently reported subclass in their series of anti-GBM nephritis (20). It has been suggested in other studies that the pathogenic antibodies in anti-GBM disease may shift over time, with disease severity possibly correlating with specific IgG subtypes (21).
Anti-GBM disease is often associated with other renal vasculitides, in which setting serological assays and renal biopsy play a major role. The renal biopsy in our patient revealed 90% glomerular involvement by crescents, the majority of which were acute (cellular), with lesser involvement by subacute (fibrocellular) and remote (fibrous) crescents. The presence of such temporal heterogeneity in crescents is unusual for anti-GBM nephritis, where all of the crescents are typically in the same acute stage.
Conclusions
Our case highlights the critical importance of renal biopsy in the diagnosis of anti-GBM disease, particularly in the absence of positive serologic testing. It also reveals the limitations of commercially available assays and the need to understand the intricacies with each methodology. Accurate recognition of the presence of anti-GBM disease is crucial for early institution of therapy, renal recovery and prognosis, and mortality in this small subset of patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and for any accompanying images.
References
- Pedchenko V, Bondar O, Fogo AB, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 2010;363:343-54. [Crossref] [PubMed]
- Kalluri R, Wilson M, Weber M, et al. Identification of the α3 chain of Type IV collagen as the common autoantigen in anti-basement membrane disease and Goodpasture syndrome. J Am Soc Nephrol 1995;6:1178-85. [PubMed]
- Salama AD, Dougan T, Levy JB, et al. Goodpasture’s disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques. Am J Kidney Dis 2002;39:1162-7. [Crossref] [PubMed]
- McAdoo SP, Pusey CD. Anti-glomerular Basement Membrane Disease. In: Mackay IR, Rose NR, Diamond B, et al. editors. Encyclopedia of Medical Immunology. New York, NY: Springer, 2014:50-6.
- Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med 1967;126:989-1004. [Crossref] [PubMed]
- Pusey CD. Anti-glomerular basement membrane disease. Kidney Int 2003;64:1535-50. [Crossref] [PubMed]
- Stolk M, Carl D, Massey H. Antibody-negative Goodpasture’s disease. NDT Plus 2010;3:253-6. [PubMed]
- Ohlsson S, Herlitz H, Lundberg S, et al. Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis 2014;63:289-93. [Crossref] [PubMed]
- Border WA, Baehler RW, Bhathena D, et al. IgA antibasement membrane disease with pulmonary haemorrhage. Ann Intern Med 1979;91:21-5. [Crossref] [PubMed]
- Jia XY, Qu Z, Cui Z, et al. Circulating anti-glomerular basement membrane autoantibodies against α3(IV)N C1 undetectable by commercially available enzyme linked immunosorbent assays. Nephrology 2012;17:160-6. [Crossref] [PubMed]
- Sinico RA, Radice A, Corace C, et al. Anti-glomerular basement membrane antibodies in the diagnosis of Goodpasture syndrome: a comparison of different assays. Nephrol Dial Transplant 2006;21:397-401. [Crossref] [PubMed]
- Mahler M, Radice A, Sinico RA, et al. Performance evaluation of a novel chemiluminescence assay for detection of anti-GBM antibodies: an international multicenter study. Nephrol Dial Transplant 2012;27:243-52. [Crossref] [PubMed]
- Wilson CB, Dixon FJ. Diagnosis of immunopathologic renal disease. Kidney Int 1974;5:389-401. [Crossref] [PubMed]
- Borza DB, Netzer KO, Leinonen A, et al. The Goodpasture autoantigen: identification of multiple cryptic chain. J Biol Chem 2000;275:6030-7. [Crossref] [PubMed]
- Hellmark T, Burkhardt H, Weislander J. Goodpasture disease: characterization of a single conformational epitope as the target of pathogenic autoantibodies. J Biol Chem 1999;274:25862-8. [Crossref] [PubMed]
- Netzer KO, Leinonen A, Boutard A, et al. The goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha residues 17-31 and 127-141 of the NC1 domain. J Biol Chem 1999;274:11267-74. [Crossref] [PubMed]
- Glassock RJ. Atypical anti-glomerular basement membrane disease: lessons learned. Clin Kidney J 2016;9:653-6. [Crossref] [PubMed]
- Nasr SH, Collins AB, Alexander MP, et al. The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int 2016;89:897-908. [Crossref]
- Liang D, Liang S, Xu F, et al. Clinic pathological features and outcome of antibody-negative anti-glomerular basement membrane disease. J Clin Pathol 2019;72:31-7. [Crossref] [PubMed]
- Qu Z, Cui Z, Liu G, et al. The distribution of IgG subclass deposition on renal tissues from patients with anti-glomerular basement membrane disease. BMC Immunol 2013;14:19. [Crossref] [PubMed]
- Gu B, Magil AB, Barbour SJ. Frequently relapsing anti-glomerular basement membrane antibody disease with changing clinical phenotype and antibody characteristics over time. Clin Kidney J 2016;9:661-4. [Crossref] [PubMed]



