
An emerging phenotype of central nervous system involvement in Pompe disease: from bench to bedside and beyond
Introduction
Pompe disease (PD) (OMIM: 232300), also known as glycogen storage disease type II (GSD II) or glycogenosis type II, is a lysosomal storage disorder caused by deficiency of the lysosomal enzyme acid-alpha glucosidase (GAA). Pathogenic variants in the GAA gene lead to deficient or absent GAA and excessive accumulation of lysosomal glycogen primarily in the heart, skeletal, smooth muscles, and the nervous system. PD encompasses a phenotypic continuum which is broadly classified into two main groups (I) infantile Pompe disease (IPD) at the severe end of the clinical spectrum and (II) late-onset Pompe disease (LOPD), which has less severe clinical outcomes (1). Children with IPD present with hypertrophic cardiomyopathy, hypotonia, and muscle weakness in the first few days to months of life. IPD also represents a continuum and is further classified into (I) classic IPD characterized by severe cardiomyopathy and death typically within the first 1–2 years of life and (II) non-classic IPD, which presents in the first year of life with less severe cardiomyopathy and a more slowly progressive myopathy (1). LOPD is primarily differentiated from IPD by the absence of cardiomyopathy in the first year of life and the presence of a more slowly progressive myopathy (2). Based on the age at onset, LOPD is further classified into (I) childhood, juvenile, or muscular variant (second year to adolescence) and (II) adult-onset LOPD (second to sixth decade) (2,3). With the approval of enzyme replacement therapy (ERT) with recombinant human GAA (rhGAA, Alglucosidase alfa) by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2006 and the subsequent success of immunomodulation protocols to prevent anti-drug antibody responses to ERT, along with newborn screening initiatives and a multidisciplinary management approach, patients with IPD are living longer and a new phenotype of survivors is emerging (4-6). This review article summarizes the CNS findings in patients, highlights CNS findings in animal models, explores gene therapy as a method of reversing CNS pathologies as reported by some breakthrough preclinical studies, and emphasizes the need to follow patients and monitor for CNS involvement over time.
CNS involvement in patients with PD
PD has long been considered a metabolic muscle disorder. While the clinical hallmarks of PD impact muscle function, it is recognized that the CNS is also involved. As early as 1965, brain autopsies demonstrated abnormal glycogen accumulation in the cerebral cortex, cerebellum, anterior horn cells, and spinal cord in patients with IPD and in the cerebral cortex and spinal cord in patients with LOPD (7-11) (see Table 1). However, to date, limited clinical data are available demonstrating the extent and clinical significance of CNS involvement in PD. As IPD survivors have aged, neurologic symptoms including sensorineural hearing loss, foot-slapping gait, bulbar weakness with dysarthria and dysphagia, and small fiber neuropathy have become apparent (6,20-22). Problems with processing speed, learning disabilities, and on rare occasions cognitive declines have also been described in some IPD patients (24,25). Patients with LOPD can also develop sensorineural hearing loss and there is some evidence of cognitive decline, mainly affecting executive functioning (26-29).

Full table
Neuroimaging with magnetic resonance imaging (MRI) or angiograms (MRA), computed tomography, and ultrasounds have been used to analyze the structural anatomic areas of the brain in patients with PD (see Table 2). Collective data from serial MRIs on 13 patients with IPD (age range: 20 months–13 years) showed that all these patients with a normal baseline MRI in infancy later developed white matter hyperintense foci (WMF) at variable rates, from as early as 6 months to as late as 10 years (30,33,34,36,37,47). Periventricular, subcortical, and deep white matter were shown to be involved in reports by various authors (30,33,34,36,37,47). Additional research is required to determine whether PD has a certain pattern of WM involvement and its impact from a clinical perspective. In addition to WMF, other structural abnormalities seen in patients with IPD include ventricular enlargement, extra axial cerebrospinal fluid, or thinning of the corpus callosum during infancy with the resolution of these findings with increasing age (34). Studies using magnetic resonance spectroscopy show neuronal injury and loss of myelination, which may be suggestive of neurodegeneration in children with IPD (31,32). In LOPD, collective data from MRIs on 3 patients (age range: 6–45 years) showed WMF in the periventricular regions and the frontal lobe of cerebrum (14,27,28). It is known that cerebral blood vessel involvement in the form of intracranial aneurysms are one of the leading causes of death in LOPD (13). Intracranial aneurysm has also been described in a 7-year-old child with IPD (39).
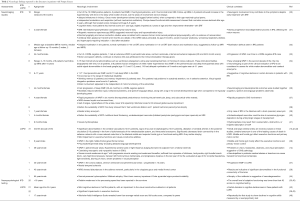
Full table
Cognitive functioning in PD can be influenced by a number of factors such as genetic variants, rate of disease progression, other medical comorbidities that affect the wellbeing of an individual, and environmental exposures. Data on cognition in PD are limited. Developmental outcomes have been assessed in patients with IPD using different age-appropriate neuropsychological tests (see Table 2). The overall profile of skills shown by those participants with below average academic skills was consistent with a learning disability diagnosis rather than an intellectual disability (24). Relative weakness in the processing speed has been reported in the survivors of IPD (35,45). Problems in certain domains of cognition such as sustained attention (n=5), visual-spatial integration (n=3), and working memory (n=5) have been reported (35) for patients with IPD. In LOPD, developmental delays in childhood have been documented in a single case report (28), and significant impairments in executive functions, visual-constructive abilities, and short-term memory have been observed in adults (n=1 to 21) (29,43,44,46). Further research is needed to better understand the extent of nervous system involvement in PD across the disease spectrum and to explore possible correlations between abnormalities detected through neuroimaging and developmental outcomes.
CNS histopathology in PD
Based on published literature, pathogenesis of muscle damage in PD has been attributed to dysregulated autophagy, lysosomal glycogen accumulation, impaired calcium homeostasis, increased reactive oxygen species, and mitochondrial defects (48). However, CNS pathogenesis is still unclear. To better understand CNS disease progression and the underlying mechanism in PD, it is important to integrate the knowledge obtained from autopsy data from patients and preclinical animal studies.
Histopathology findings in patients with PD
The major histopathological changes seen in patients with PD are due to extensive glycogen accumulation in the brain, spinal cord, and anterior horn cells (see Table 1). Autopsy reports of patients with IPD have shown abnormal glycogen accumulation in the neurons of the cerebral cortex, midbrain, pons, medulla, cranial nerve nuclei, Schwann cells, glial cells, astrocytes throughout the brainstem and cerebellum (13). Vacuolization was seen in the medulla, certain nuclei in the brain, and anterior horn cells (12,13). Gliosis has also been seen in patients with IPD (12). The most prominent histological finding in patients with LOPD was the involvement of the cerebral vasculature showing vacuolization, scant glycogen in cerebellar pericytes, and aneurysms in the large, medium, and small arteries of the brain (10,14,15). In addition, astrogliosis was seen in the cerebrum and cerebellum on autopsy of a 40-year-old male patient with LOPD (14). The patient had myelin abnormalities, ranging from focal areas of demyelination to necrosis in the brain (14) (Table 2 for clinical details). These histopathological findings, seen in both IPD and LOPD, point to what could be the cause for the development of WMF, namely vasculopathies causing ischemia and small cerebral artery damages, gliosis, and myelin changes (49) (Tables 1,2).
Normal myelination requires a close association between glial cells (oligodendrocytes, astrocytes, and Schwann cells) and neurons. In IPD, glycogen accumulation was seen in oligodendrocytes in the second trimester of gestation (9). As described above, autopsy reports of IPD show that astrocytes, Schwann cells, and neurons in the anterior horn cells and cortex had glycogen accumulation, along with neuronal losses in the brain and spinal cord (7,10,13). Therefore, abnormalities in these cells could be the cause of problems in the white matter integrity (such as de-/dys-myelination) in patients with IPD (50). In children with IPD, studies using magnetic resonance spectroscopy have described neuronal injury and loss of myelination, which may be suggestive of neurodegeneration (31,32).
Histopathology findings in spontaneous animal models of PD
Studies of naturally occurring animal models of PD have been reported in cats, sheep, cattle, dogs, and quail (Table 3). GAA deficiency was not tested in the cat and sheep models but the affected animals exhibit pathological phenotypes that closely resemble human PD. Electron microscopy analysis showed the presence of large amounts of rounded glycogen-loaded bodies that are limited by a single membrane in both the nerve and glial cells in the spinal medulla of the affected cats (51). In the diseased sheep, neurons in the midbrain, brain stem, cerebellum, and dorsal root ganglia were heavily affected with PAS-positive granules. Electron microscopy image showed that the affected neurons contained both empty vacuoles and lysosome-like structures filled with granular material resembling glycogen (52).
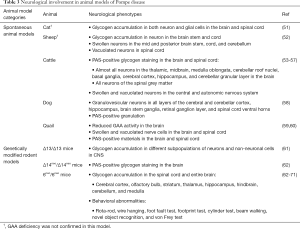
Full table
Several reports have described CNS involvement in cattle (Table 3). Glycogen content was increased and GAA activity reduced in both the brain and spinal cord of affected cattle (56). Swollen neurons containing PAS-positive vacuoles were seen throughout the CNS (53-56). In particular, many neurons in the cerebral cortex, mid-brain thalamus, hippocampus, brain stem, roof nuclei, molecular and granular layers of the cerebellum, and medulla were found to be affected (53,56). Glial cells in the white matter of the cerebrum and cerebellum were enlarged and contained PAS-positive glycogen (56). Purkinje cells also contained PAS-positive granules but were rarely swollen or vacuolated (53,56).
Similar to the cattle model, a dog model of PD revealed the presence of PAS-positive granulated neurons in the cerebral and cerebellar cortex, hippocampus, brain stem ganglia, retinal ganglion layer, and spinal cord ventral horns (58). Japanese quail, once used in the preclinical studies before the development of mouse models, showed decreased GAA enzyme activity in the brain and spinal cord on necropsy (60). Histological results showed that nerve cells were swollen and vacuolated and the accumulation of PAS-positive glycogen was observed in the brain and spinal cord (59).
Collective data from these naturally occurring animal models seem consistent with CNS findings in patients with PD.
Histopathology findings in rodent models of PD
Mice share many common genetic features with humans and mouse models are extremely useful for studying human disease and testing new therapies. To date, four genetically modified knockout mouse (GAAKO) models of PD have been reported. The first mouse model (Δ13/Δ13) was generated by disrupting exon13 in the Gaa gene (72); the second is 6neo/6 neo, in which the Gaa gene reading frame is disrupted by inserting a neomycin-resistance gene (73); the third is Δ6/Δ6, in which exon 6 was deleted from the Gaa gene by Cre/lox-mediated recombination (73); and the fourth model, Δ14neo/Δ14neo, was created by a targeted deletion of exon 14 (62). Unlike in humans where disease severity and age of onset are closely associated with the level of residual GAA activity, all these knockout mouse models had undetectable GAA enzyme activity but variable disease severity. All four mouse models accumulated glycogen in the heart, skeletal muscles, and the brain, however, only the 6neo/6neo and Δ14neo/Δ14neo mice showed a severe phenotype by developing early functional impairments of mobility and muscle strength (62,73). The 6neo/6neo model resembles critical features of both the infantile and adult forms of the human disease and has been the most used animal model for pre-clinical therapy development over the past 20 years (62,73). CNS involvement in GAAKO mice have been reported in the Δ13/Δ13, 6neo/6neo, and Δ14neo/Δ14neo mouse models (Table 3).
Recent studies have shown progressive glycogen accumulation in the CNS of GAAKO mice by PAS-staining of tissue sections. Noticeable glycogen accumulation was observed in the brain and spinal cord of 2-week-old GAAKO mice and PAS-positive glycogen was even detected in some motor neurons in the brainstem on the first day after birth (70,71). At 1 month of age, still asymptomatic, GAAKO mice showed a moderate accumulation of glycogen in the entire brain and spinal cord, which continued to increase over time (64,70,71). By 3 months of age, there was a striking increase in the amount of accumulated glycogen, and by 12 months the pathology could be characterized as extensive (71).
In the brain, glycogen accumulation occurs in both neurons and glial cells in the cerebral cortex, corpus callosum, and hippocampus (64,71). A dramatic increase in glycogen accumulation was seen in the granule cell neurons in the glomerular layer of the olfactory bulb (64,71). In the cerebellum, massive glycogen accumulation was observed in the Golgi epithelial cells in the cortex, in the granule cells, and the cytoplasm of most glial cells in the cerebellar cortical white matter (63,64,70,71). In brainstem (mainly medulla) and spinal cord, PAS staining revealed extensive glycogen accumulation in the large neurons, especially in the motoneurons (also known as motor neurons) (64,68-71). Swollen degenerating axons and motoneurons containing numerous vacuoles were also found in these regions (64,68).
In the GAAKO mice, neurodegeneration and neuroinflammation were detected in the brain by immunohistochemical markers, but showed no evidence of a correlation with the glycogen accumulation (68). Transcriptome analysis of the spinal cord revealed that both the cell death and proinflammatory signaling pathways were dramatically upregulated. TUNEL (transferase dUTP nick end labeling) assay confirmed the progressive cell death in the spinal cord of GAAKO mice (69). Astrogliosis was observed in some old GAAKO mice and asymptomatic young mice particularly in the white matter of spinal cord (64,68). Microglial activation was noted in the spinal cord of GAAKO mice even at a young age (at 6 weeks) (68).
The age-wise, progressive glycogen accumulation in the CNS observed in the GAAKO mouse models may be helpful to better understand the underlying disease progression and identify novel endpoints in humans since there are very few longitudinal studies in patients with PD that explore the extent of CNS lesions and its impact on clinical outcomes. Neurodegeneration described here also requires to be translated to clinical studies in patients with PD.
Defects of neurological functions in GAAKO mice
Functional tests including grip strength, wire hang, treadmill, and rotarod tests that have been used in most preclinical studies with GAAKO mice were mainly used to measure skeletal muscle strength, and did not define the role of a neurological component, if any, in the overall neuromuscular dysfunction. Respiratory dysfunction was previously considered a consequence of muscle weakness, but recent studies have suggested that respiratory dysfunction may be a measure of neurological impairment, which can also contribute to the respiratory problems in PD (65). The muscle-specific transgenic GAAKO mice expressing hGAA only in skeletal muscle showed a normal functioning diaphragm muscle. Even with normal diaphragm function, the transgenic mice still showed a similar abnormal ventilation pattern similar to the GAAKO mice during quiet breathing without improvement, suggesting a neurologic rather than muscle etiology (65). Furthermore, glycogen accumulation was detected in the respiratory-related motoneurons in GAAKO mice (65,68).
We recently performed a comprehensive set of behavioral tests to evaluate the impairments of motor coordination and balance, sensation, and memory in GAAKO mice in an attempt to understand the association of glycogen accumulation in the CNS with neurological defects (71). Neurological defects in muscle coordination and balance were demonstrated by four tests: the GAAKO mice showed shorter latency to fall in the rotarod test, asymmetric forelimb usage in the cylinder test, increased time to cross the narrow beam in the beam walking test, and gait abnormalities in the footprint test in comparison with age-matched wild-type (WT) mice. Sensory impairment was tested by the von Frey test, which showed that the withdrawal threshold increased dramatically in the GAAKO mice compared with the WT controls. Impairments in memory were assessed by the novel object recognition test and it showed that GAAKO mice spent less time exploring a novel object than the WT mice (71). These results strongly support that there is neurologic involvement in GAAKO mice and provide a quantitative method for evaluating CNS functioning in preclinical studies. We successfully used these behavioral tests to evaluate the effect of gene therapy on the correction of muscle and CNS defects in GAAKO mice (71).
Recent development of CNS-targeted gene therapy for PD
ERT with rhGAA has no effect on the CNS in patients with PD due to its inability to cross the blood-brain barrier (BBB) (74). In contrast, adeno-associated virus (AAV)-mediated gene therapy may provide an improved treatment option for PD due to the availability of novel AAV serotypes such as (75,76) AAV serotype 9 (AAV9) that can transduce muscle tissues with high efficiency and cross the BBB to deliver the therapeutic gene to the CNS (75-80). In the past 15 years, numerous studies have demonstrated the efficacy of gene therapy with AAV vectors expressing hGAA in GAAKO mice, however most prior studies focused on the correction of skeletal and cardiac abnormalities. Recently great efforts have been made to improve the correction of the CNS abnormalities in GAAKO mice using selective AAV serotypes, neuron-specific promoters, and various vector injection routes. In this review, we will focus on the recent articles related to CNS-targeted gene therapy in GAAKO mice (Table 4).
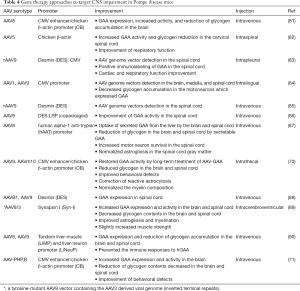
Full table
Several studies have demonstrated that administration of AAV vectors directly into the brain or spinal cord can effectively correct glycogen storage in the CNS but not in muscles of GAAKO mice (70,89). Intraspinal injection of an AAV5 vector expressing hGAA restored GAA activity and cleared neuronal glycogen accumulation in the region of spinal cord near the injection site and improved ventilation in GAAKO mice (82). Intrathecal administration of an AAVrh10- or AAV9-CAG-hGAA vector into GAAKO mice effectively reduced glycogen accumulation in the brain and spinal cord and achieved sustained neurologic and neuromuscular correction (70). Intracerebroventricular injection of a tyrosine-mutant AAV9/3 vector containing a neuronal cell-specific promoter to drive hGAA expression into neonatal GAAKO mice cleared the accumulated glycogen in the brain and spinal cord, but not in the muscle. Gene therapy with this vector substantially improved rotarod performance even though the strength of skeletal muscle was only slightly increased (89).
A recent study used a novel tandem promoter (LiNeuP) comprising a hepatocyte-specific promoter (hATT) and a neuron-specific (hSYN) promoter to prevent hGAA-induced immune responses in GAAKO mice (90). This study used an intravenous injection of the tolerogenic AAV9-LiNeuP-hGAA vector in GAAKO mice, which resulted in persistent hGAA expression in the liver and the CNS without provoking anti-hGAA immune responses. However, this treatment corrected glycogen storage only in the CNS and liver and had no effect on the skeletal muscles (90).
AAVB1 is a newly engineered vector that showed higher efficiency than AAV9 in transducing the brain, spinal cord, and muscle in mice (91). Intravenous administration of an AAVB1-GAA vector in GAAKO mice resulted in a significant increase in vector transduction in the thoracic spinal cord, compared to the AAV9-GAA treatment. However, the two treatments showed a similar level of vector transduction in the medulla, cervical, and lumbar spinal cord (88).
We recently reported that a single intravenous injection of a novel AAV-PHP.B vector expressing hGAA into 2-week-old GAAKO mice resulted in widespread GAA expression in all the affected tissues including the CNS (71). Glycogen contents were reduced to WT levels in the brain and heart, and significantly decreased in the skeletal muscles by the AAV treatment. Histology showed no visible glycogen accumulation in any region of the brain and spinal cord of the AAV-treated mice. Improvement of neuromuscular and neurological function was demonstrated following the AAV treatment (71). This is the first study to demonstrate that the reduction of glycogen in the CNS can improve neurological functions in PD (71). Despite these promising results in mice, recent studies showed that the AAV-PHP.B vector failed to robustly transduce the CNS in WT primates (non-human animal models) (92,93). With the rapid advancement of AAV vector technology, development of a novel AAV vector that can transduce both muscle and the CNS in humans is vital for effective gene therapy. At this point, more studies are warranted in animal models to allow for clinical translation into patients.
Conclusions
Until recently, PD was considered a muscle disorder due to deficiency of acid alfa glucosidase. With advances in research and availability of ERT, patients with PD are living longer. A new phenotype is emerging across the disease spectrum among the survivors. It is now clear that there is a neurological component to the disease. Data from animal studies and clinical research suggest that the functional deficits observed in patients with PD are attributed to dysfunction of both muscle and the nervous system. However, the true extent of CNS pathology and its clinical impact is still not well understood. Hence, there is need for a comprehensive approach, which includes detailed neurological examination, routine neuroimaging and measurement of developmental outcomes, to monitor patients over time. Animal models provide a valuable tool for understanding disease progression in the CNS and for exploring novel therapies for PD. Our data from GAAKO mice demonstrate that AAV-mediated gene therapy improves neurological defects, but additional research is necessary to translate these findings into human trials (71). Delineation of CNS involvement in patients with PD, through systematic integration of the knowledge obtained from animal models and clinical studies in patients, could enable development of successful therapies that can specifically target CNS pathology and improve outcomes.
Acknowledgments
The authors would like to thank Heidi Cope for her valuable inputs during the review process of the manuscript.
Footnote
Conflicts of Interest: PS Kishnani has received research/grant support from Sanofi Genzyme, Valerion Therapeutics, and Amicus Therapeutics. She has received consulting fees and honoraria from Sanofi Genzyme, Amicus Therapeutics, Vertex Pharmaceuticals and Asklepios Biopharmaceutical, Inc. (AskBio). She is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme, Amicus Therapeutics, and Baebies. She has equity in AskBio, which is developing gene therapy for Pompe disease. The other authors have no conflicts of interest to declare.
References
- Kishnani PS, Steiner RD, Bali D, et al. Pompe disease diagnosis and management guideline. Genet Med 2006;8:267-88. [Crossref] [PubMed]
- Chan J, Desai AK, Kazi ZB, et al. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol Genet Metab 2017;120:163-72. [Crossref] [PubMed]
- Reuser AJ, Hirschhorn R, Kroos MA. Glycogen Storage Disease Type II, Acid α-Glucosidase (Acid Maltase) Deficiency. In: Valle D, Beaudet AL, Vogelstein B, et al. editors. The Online Metabolic and Molecular Bases of Inherited Disease. 8th edition. New York, NY: McGraw-Hill Medical Publishing Division, 2001.
- Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007;68:99-109. [Crossref] [PubMed]
- Kazi ZB, Prater SN, Kobori JA, et al. Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses. JCI Insight 2016;1:e86821. [Crossref] [PubMed]
- Prater SN, Banugaria SG, DeArmey SM, et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med 2012;14:800-10. [Crossref] [PubMed]
- Martini C, Ciana G, Benettoni A, et al. Intractable fever and cortical neuronal glycogen storage in glycogenosis type 2. Neurology 2001;57:906-8. [Crossref] [PubMed]
- Mancall EL, Aponte GE, Berry RG. Pompe's Disease (Diffuse Glycogenosis) with Neuronal Storage. J Neuropathol Exp Neurol 1965;24:85-96. [Crossref] [PubMed]
- Gambetti P, DiMauro S, Baker L. Nervous system in Pompe's disease. Ultrastructure and biochemistry. J Neuropathol Exp Neurol 1971;30:412-30. [Crossref] [PubMed]
- Hobson-Webb LD, Proia AD, Thurberg BL, et al. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab 2012;106:462-9. [Crossref] [PubMed]
- Thurberg BL, Lynch Maloney C, Vaccaro C, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest 2006;86:1208-20. [Crossref] [PubMed]
- Teng YT, Su WJ, Hou JW, et al. Infantile-onset glycogen storage disease type II (Pompe disease): Report of a case with genetic diagnosis and pathological findings. Chang Gung Med J 2004;27:379-84. [PubMed]
- Pena LD, Proia AD, Kishnani PS. Postmortem Findings and Clinical Correlates in Individuals with Infantile-Onset Pompe Disease. JIMD Rep 2015;23:45-54. [Crossref] [PubMed]
- Kretzschmar HA, Wagner H, Hübner G, et al. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci 1990;98:169-83. [Crossref] [PubMed]
- Miyamoto Y, Etoh Y, Joh R, et al. Adult-onset acid maltase deficiency in siblings. Acta Pathol Jpn 1985;35:1533-42. [PubMed]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005;7:452-64. [Crossref] [PubMed]
- Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010;68:409-27. [Crossref] [PubMed]
- Martin JJ, de Barsy T, Van Hoof F, et al. Pompe's disease: An inborn lysosomal disorder with storage of glycogen. Acta Neuropathol 1973;23:229-44. [Crossref] [PubMed]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010;119:7-35. [Crossref] [PubMed]
- Hobson-Webb LD, Austin SL, Jain S, et al. Small-fiber neuropathy in pompe disease: first reported cases and prospective screening of a clinic cohort. Am J Case Rep 2015;16:196-201. [Crossref] [PubMed]
- Jones HN, Muller CW, Lin M, et al. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia 2010;25:277-83. [Crossref] [PubMed]
- van Capelle CI, Goedegebure A, Homans NC, et al. Hearing loss in Pompe disease revisited: results from a study of 24 children. J Inherit Metab Dis 2010;33:597-602. [Crossref] [PubMed]
- Prakalapakorn SG, Proia AD, Yanovitch TL, et al. Ocular and histologic findings in a series of children with infantile pompe disease treated with enzyme replacement therapy. J Pediatr Ophthalmol Strabismus 2014;51:355-62. [Crossref] [PubMed]
- Spiridigliozzi GA, Keeling LA, Stefanescu M, et al. Cognitive and academic outcomes in long-term survivors of infantile-onset Pompe disease: A longitudinal follow-up. Mol Genet Metab 2017;121:127-37. [Crossref] [PubMed]
- Ebbink BJ, Poelman E, Plug I, et al. Cognitive decline in classic infantile Pompe disease: An underacknowledged challenge. Neurology 2016;86:1260-1. [Crossref] [PubMed]
- Musumeci O, Catalano N, Barca E, et al. Auditory system involvement in late onset Pompe disease: a study of 20 Italian patients. Mol Genet Metab 2012;107:480-4. [Crossref] [PubMed]
- Montagnese F, Barca E, Musumeci O, et al. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J Neurol 2015;262:968-78. [Crossref] [PubMed]
- Guevara-Campos J, González-Guevara L, Cauli O. Skeletal alterations, developmental delay and new mutations in juvenile-onset Pompe's disease. Neuromuscul Disord 2019;29:192-7. [Crossref] [PubMed]
- Borroni B, Cotelli MS, Premi E, et al. The brain in late-onset glycogenosis II: a structural and functional MRI study. J Inherit Metab Dis 2013;36:989-95. [Crossref] [PubMed]
- Chien YH, Lee NC, Chen CA, et al. Long-term prognosis of patients with infantile-onset Pompe disease diagnosed by newborn screening and treated since birth. J Pediatr 2015;166:985-91.e1-2.
- Burrow TA, Bailey LA, Kinnett DG, et al. Acute progression of neuromuscular findings in infantile Pompe disease. Pediatr Neurol 2010;42:455-8. [Crossref] [PubMed]
- Chien YH, Lee NC, Peng SF, et al. Brain development in infantile-onset Pompe disease treated by enzyme replacement therapy. Pediatr Res 2006;60:349-52. [Crossref] [PubMed]
- Messinger YH, Mendelsohn NJ, Rhead W, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med 2012;14:135-42. [Crossref] [PubMed]
- McIntosh PT, Hobson-Webb LD, Kazi ZB, et al. Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy. Mol Genet Metab 2018;123:85-91. [Crossref] [PubMed]
- Ebbink BJ, Poelman E, Aarsen FK, et al. Classic infantile Pompe patients approaching adulthood: a cohort study on consequences for the brain. Dev Med Child Neurol 2018;60:579-86. [Crossref] [PubMed]
- Rohrbach M, Klein A, Köhli-Wiesner A, et al. CRIM-negative infantile Pompe disease: 42-month treatment outcome. J Inherit Metab Dis 2010;33:751-7. [Crossref] [PubMed]
- Broomfield A, Fletcher J, Hensman P, et al. Rapidly Progressive White Matter Involvement in Early Childhood: The Expanding Phenotype of Infantile Onset Pompe? JIMD Rep 2018;39:55-62. [Crossref] [PubMed]
- van den Hout HM, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003;112:332-40. [Crossref] [PubMed]
- Patel TT, Banugaria SG, Frush DP, et al. Basilar artery aneurysm: a new finding in classic infantile Pompe disease. Muscle Nerve 2013;47:613-5. [Crossref] [PubMed]
- Lee CC, Chen CY, Chou TY, et al. Cerebral MR manifestations of Pompe disease in an infant. AJNR Am J Neuroradiol 1996;17:321-2. [PubMed]
- Sahin M, du Plessis AJ. Hydrocephalus associated with glycogen storage disease type II (Pompe's disease). Pediatr Neurol 1999;21:674-6. [Crossref] [PubMed]
- Hensel O, Hanisch F, Stock K, et al. Morphology and Function of Cerebral Arteries in Adults with Pompe Disease. JIMD Rep 2015;20:27-33. [Crossref] [PubMed]
- Musumeci O, Marino S, Granata F, et al. Central Nervous System involvement in Late Onset Pompe Disease (LOPD): clues from neuroimaging and neuropsychological analysis. Eur J Neurol 2019;26:442-e35. [Crossref] [PubMed]
- D'Amico MC, Di Tommaso V, Di Giacomo R, et al. A Case of Normal Pressure Hydrocephalus in Adult-Onset Pompe Disease. J Neuromuscul Dis 2015;2:S14. [PubMed]
- Spiridigliozzi GA, Heller JH, Kishnani PS. Cognitive and adaptive functioning of children with infantile Pompe disease treated with enzyme replacement therapy: long-term follow-up. Am J Med Genet C Semin Med Genet 2012;160c:22-9. [Crossref] [PubMed]
- Muraoka T, Murao K, Imachi H, et al. Novel mutations in the gene encoding acid alpha-1,4-glucosidase in a patient with late-onset glycogen storage disease type II (Pompe disease) with impaired intelligence. Intern Med 2011;50:2987-91. [Crossref] [PubMed]
- Ebbink BJ, Aarsen FK, van Gelder CM, et al. Cognitive outcome of patients with classic infantile Pompe disease receiving enzyme therapy. Neurology 2012;78:1512-8. [Crossref] [PubMed]
- Kohler L, Puertollano R, Raben N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018;15:928-42. [Crossref] [PubMed]
- Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008;64:273-80. [Crossref] [PubMed]
- Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 2009;72:750-9. [Crossref] [PubMed]
- Sandstrom B, Westman J, Ockerman PA. Glycogenosis of the central nervous system in the cat. Acta Neuropathol 1969;14:194-200. [Crossref] [PubMed]
- Manktelow BW, Hartley WJ. Generalized glycogen storage disease in sheep. J Comp Pathol 1975;85:139-45. [Crossref] [PubMed]
- Richards RB, Edwards JR, Cook RD, et al. Bovine generalized glycogenosis. Neuropathol Appl Neurobiol 1977;3:45-56. [Crossref]
- Howell JM, Dorling PR, Cook RD, et al. Infantile and late onset form of generalised glycogenosis type II in cattle. J Pathol 1981;134:267-77. [Crossref] [PubMed]
- O'Sullivan BM, Healy PJ, Fraser IR, et al. Generalised glycogenosis in Brahman cattle. Aust Vet J 1981;57:227-9. [Crossref] [PubMed]
- Cook RD, Howell JM, Dorling PR, et al. Changes in nervous tissue in bovine generalized glycogenosis type II. Neuropathol Appl Neurobiol 1982;8:95-107. [Crossref] [PubMed]
- Lyons RE, Johnston DJ, McGowan MR, et al. E7 (1057DeltaTA) mutation of the acidic alpha-glucosidase gene causes Pompe's disease in Droughtmaster cattle. Aust Vet J 2017;95:138-42. [Crossref] [PubMed]
- Walvoort HC, Dormans JA, van den Ingh TS. Comparative pathology of the canine model of glycogen storage disease type II (Pompe's disease). J Inherit Metab Dis 1985;8:38-46. [Crossref] [PubMed]
- Matsui T, Kuroda S, Mizutani M, et al. Generalized glycogen storage disease in Japanese quail (Coturnix coturnix japonica). Vet Pathol 1983;20:312-21. [Crossref] [PubMed]
- Kikuchi T, Yang HW, Pennybacker M, et al. Clinical and metabolic correction of pompe disease by enzyme therapy in acid maltase-deficient quail. J Clin Invest 1998;101:827-33. [Crossref] [PubMed]
- Bijvoet AG, Van Hirtum H, Vermey M, et al. Pathological features of glycogen storage disease type II highlighted in the knockout mouse model. J Pathol 1999;189:416-24. [Crossref] [PubMed]
- Raben N, Nagaraju K, Lee E, et al. Modulation of disease severity in mice with targeted disruption of the acid alpha-glucosidase gene. Neuromuscul Disord 2000;10:283-91. [Crossref] [PubMed]
- Taksir TV, Griffiths D, Johnson J, et al. Optimized preservation of CNS morphology for the identification of glycogen in the Pompe mouse model. J Histochem Cytochem 2007;55:991-8. [Crossref] [PubMed]
- Sidman RL, Taksir T, Fidler J, et al. Temporal neuropathologic and behavioral phenotype of 6neo/6neo Pompe disease mice. J Neuropathol Exp Neurol 2008;67:803-18. [Crossref] [PubMed]
- DeRuisseau LR, Fuller DD, Qiu K, et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A 2009;106:9419-24. [Crossref] [PubMed]
- Lee KZ, Qiu K, Sandhu MS, et al. Hypoglossal neuropathology and respiratory activity in pompe mice. Front Physiol 2011;2:31. [Crossref] [PubMed]
- Fuller M, Duplock S, Turner C, et al. Mass spectrometric quantification of glycogen to assess primary substrate accumulation in the Pompe mouse. Anal Biochem 2012;421:759-63. [Crossref] [PubMed]
- Turner SM, Hoyt AK, ElMallah MK, et al. Neuropathology in respiratory-related motoneurons in young Pompe (Gaa(-/-)) mice. Respir Physiol Neurobiol 2016;227:48-55. [Crossref] [PubMed]
- Turner SMF, Falk DJ, Byrne BJ, et al. Transcriptome assessment of the Pompe (Gaa-/-) mouse spinal cord indicates widespread neuropathology. Physiol Genomics 2016;48:785-94. [Crossref] [PubMed]
- Hordeaux J, Dubreil L, Robveille C, et al. Long-term neurologic and cardiac correction by intrathecal gene therapy in Pompe disease. Acta Neuropathol Commun 2017;5:66. [Crossref] [PubMed]
- Lim JA, Yi H, Gao F, et al. Intravenous Injection of an AAV-PHP.B Vector Encoding Human Acid alpha-Glucosidase Rescues Both Muscle and CNS Defects in Murine Pompe Disease. Mol Ther Methods Clin Dev 2019;12:233-45. [Crossref] [PubMed]
- Bijvoet AG, van de Kamp EH, Kroos MA, et al. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum Mol Genet 1998;7:53-62. [Crossref] [PubMed]
- Raben N, Nagaraju K, Lee E, et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998;273:19086-92. [Crossref] [PubMed]
- Macauley SL, Sands MS. Promising CNS-directed enzyme replacement therapy for lysosomal storage diseases. Exp Neurol 2009;218:5-8. [Crossref] [PubMed]
- Gao GP, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 2002;99:11854-9. [Crossref] [PubMed]
- Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 2012;20:699-708. [Crossref] [PubMed]
- Ruzo A, Marco S, Garcia M, et al. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther 2012;23:1237-46. [Crossref] [PubMed]
- Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006;14:45-53. [Crossref] [PubMed]
- Zincarelli C, Soltys S, Rengo G, et al. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073-80. [Crossref] [PubMed]
- Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012;23:382-9. [Crossref] [PubMed]
- Sun B, Zhang H, Benjamin DK Jr, et al. Enhanced efficacy of an AAV vector encoding chimeric, highly secreted acid alpha-glucosidase in glycogen storage disease type II. Mol Ther 2006;14:822-30. [Crossref] [PubMed]
- Qiu K, Falk DJ, Reier PJ, et al. Spinal delivery of AAV vector restores enzyme activity and increases ventilation in Pompe mice. Mol Ther 2012;20:21-7. [Crossref] [PubMed]
- Falk DJ, Mah CS, Soustek MS, et al. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther 2013;21:1661-7. [Crossref] [PubMed]
- Elmallah MK, Falk DJ, Nayak S, et al. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice. Mol Ther 2014;22:702-12. [Crossref] [PubMed]
- Falk DJ, Soustek MS, Todd AG, et al. Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice. Mol Ther Methods Clin Dev 2015;2:15007. [Crossref] [PubMed]
- Doerfler PA, Todd AG, Clement N, et al. Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease. Hum Gene Ther 2016;27:43-59. [Crossref] [PubMed]
- Puzzo F, Colella P, Biferi MG, et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid alpha-glucosidase. Sci Transl Med 2017. [Crossref] [PubMed]
- Keeler AM, Zieger M, Todeasa S, et al. Systemic delivery of AAVB1-GAA clears glycogen and prolongs survival in a mouse model of Pompe disease. Hum Gene Ther 2019;30:57-68. [Crossref] [PubMed]
- Lee NC, Hwu WL, Muramatsu SI, et al. A Neuron-Specific Gene Therapy Relieves Motor Deficits in Pompe Disease Mice. Mol Neurobiol 2018;55:5299-309. [Crossref] [PubMed]
- Colella P, Sellier P, Costa Verdera H, et al. AAV Gene Transfer with Tandem Promoter Design Prevents Anti-transgene Immunity and Provides Persistent Efficacy in Neonate Pompe Mice. Mol Ther Methods Clin Dev 2018;12:85-101. [Crossref] [PubMed]
- Choudhury SR, Fitzpatrick Z, Harris AF, et al. In Vivo Selection Yields AAV-B1 Capsid for Central Nervous System and Muscle Gene Therapy. Mol Ther 2016;24:1247-57. [Crossref] [PubMed]
- Hordeaux J, Wang Q, Katz N, et al. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol Ther 2018;26:664-8. [Crossref] [PubMed]
- Matsuzaki Y, Konno A, Mochizuki R, et al. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci Lett 2018;665:182-8. [Crossref] [PubMed]


