Contemporary approach to the patient with malignant pleural effusion complicating lung cancer
Introduction
According to American Cancer Society estimates, lung cancer remained the deadliest malignancy in the United States in 2018 (1). It is the second most prevalent cancer in men and women and the commonest cause of a malignant pleural effusion (MPE), accounting for approximately 30% of such effusions (2). The course of about 15% of patients with non-small cell lung cancer (NSCLC) is complicated by a documented MPE (3). The presence of an MPE in the setting of lung cancer indicates stage IV disease and is an independent determinant of shorter survival (4). The majority of lung cancer patients with an MPE will require a pleural intervention for the palliation of dyspnea, so the identification of pleural involvement can have both diagnostic and therapeutic implications. MPE associated with lung cancer has, therefore, been at the forefront of recent advances in the diagnosis and management of pleural malignancy. The aim of the present review is to summarize the contemporary approach to the diagnosis and management of MPE in the patient with lung cancer.
Pathophysiology
Under normal conditions, the rate of pleural fluid elimination via lymphatic channels far exceeds the rate of its production at the level of pleural capillaries. A dramatic disturbance in one or both of these processes is required for clinically apparent pleural fluid accumulation to ensue. Metastatic pleural malignancy causes effusion formation by disrupting fluid circulation on both ends. Tumor deposits on the pleural surface can mechanically obstruct lymphatic drainage, leading to reduced fluid elimination (5). Concurrently, the constituent cancer cells are capable of releasing potent mediators of vascular permeability, primary among them vascular endothelial growth factor (VEGF), thereby increasing pleural capillary leak and fluid formation. It is important to recognize that pleural fluid homeostasis in patients with lung cancer can be altered by cancer-related factors other than direct tumor implantation, resulting in a so-called “paramalignant effusion”. For example, a small to moderate exudative pleural effusion may be caused by an associated pulmonary embolism, whereas the finding of a unilateral transudative effusion would prompt consideration of unexpandable lung due to malignant endobronchial obstruction.
Diagnosis
Imaging
Initial concern about possible MPE is typically generated by thoracic imaging. Certain features on contrast-enhanced computed tomography (CT) of the chest such as pleural thickening (6) and focal pleural or lung parenchymal lesions (7) heighten suspicion for pleural malignancy. However, even expert radiologists’ chest CT interpretations carry an unacceptably low negative predictive value of 65% for pleural malignancy, indicating that CT suffers from a high false-negative rate (8). The sensitivity of CT for MPE can be increased by its integration with 18Fluorodeoxyglucose positron emission tomography (18FDG-PET), reaching 93% in comparison to 70% of CT alone (9). However, the utility of 18FDG-PET is limited by problems with specificity, which was roughly 75% in a pooled analysis (10). Ultimately, regardless of the presence or absence of suggestive findings, no radiological study can substitute for cytohistological confirmation if pleural malignancy is a consideration.
Pleural fluid characteristics
Thoracentesis is the initial diagnostic intervention in cases of suspected pleural malignancy. Malignant pleural fluid is frequently, though not always, hemorrhagic (11). The prototypical MPE fulfills Light’s criteria for an exudate and exhibits a predominance of lymphocytes on cellular differential count. Besides malignancy, other etiologies associated with this pattern include pleural tuberculosis, connective tissue diseases, and coronary artery bypass grafting surgery. When present, fluid pH <7.30 is a marker of increased pleural tumor burden and therefore portends a worse prognosis and reduced pleurodesis success in MPE (12).
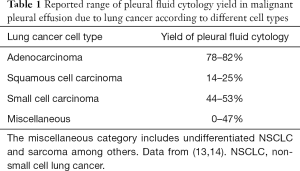
Pleural fluid cytology
The primary utility of thoracentesis in the diagnosis of MPE is the acquisition of pleural fluid for cytology. The yield of pleural fluid cytology in MPE overall is poor—about 50%—and recent studies have cast doubt on traditional dogma that repeat sampling should increase sensitivity (13,14). Adenocarcinoma is the likeliest lung cancer cell type to generate an MPE (3), and it is also associated with the highest cytological yield of the major lung cancers owing to its exfoliative nature. Pleural fluid cytology is positive in about 80% of MPE due to lung adenocarcinoma. Squamous cell carcinoma, on the other hand, has a particularly poor pleural fluid cytological yield of 25% or less (13,14). Small cell carcinoma has historically been a relatively rare cause of a clinically significant MPE (15), and it exhibits an intermediate yield of pleural fluid cytology: approximately 50%. Thus, in case of an initial negative pleural fluid cytology and ongoing concern for MPE from lung cancer, the decision between repeating cytology and proceeding to histological sampling (see below) could depend on the known or suspected cell type of the primary tumor (Table 1).
Full table
Pleural fluid tumor markers
Soluble tumor markers cannot substitute for cytohistological confirmation in MPE because they do not provide the pathological material required for the initial diagnosis of cancer and for oncological decision-making in cases of established malignancy. Measuring soluble tumor markers in pleural fluid suspected to be malignant would be of value, however, if—alone or in combination—they were more sensitive than pleural fluid cytology, thus justifying persistent concern for MPE in cytologically negative lymphocyte-predominant exudative effusions. It has been reasonably argued that the cutoff value of tumor markers must be set at the specificity level of 100% to prevent the unacceptable occurrence of false positivity (16). Applying this threshold principle, elevation of either carcinoembryonic antigen (CEA) or cancer antigen 15-3 (CA15-3) in pleural fluid has been shown to correctly classify over 40% of cytologically negative nonpurulent exudative effusions as MPE (17). The additive value of CEA and CA15-3 above pleural fluid cytology appears to vary with the histological subtype of lung cancer. In adenocarcinoma, the combined sensitivity of these tumor markers is comparable to that of pleural fluid cytology: approximately 70%. In squamous cell carcinoma, notorious for poor cytological yield, their sensitivity of 44% represents a substantial improvement over pleural fluid cytology. Although not a specific tumor marker, pleural fluid VEGF can detect malignancy in 60% of cytologically negative MPE at a threshold of 652 pg/mL (18). That cutoff is accompanied by false positivity, however, as evidenced by a specificity of 83%.
Needle pleural biopsy
If pleural fluid cytology fails to establish malignancy and proof of MPE is needed in the setting of known or suspected lung cancer, the next step is some form of pleural biopsy for histological diagnosis. Because of the patchy pleural involvement typical of metastatic implants, the yield of traditional percutaneous blunt needle biopsy of the pleura without the application of imaging (i.e., “blind” pleural biopsy) has been uniformly <50% across studies (19-21) and inferior to pleural fluid cytology in studies comparing the two (19,20). In a prospective randomized trial in which the predominant pleural malignancy was mesothelioma, dynamic CT-guided cutting needle biopsy aimed at the site of maximal pleural thickening was significantly more sensitive than the “blind” blunt needle technique: 87% vs. 47%, respectively (21). The use of static (i.e., not real-time) ultrasound (US) to assist needle biopsy of the pleura in 47 patients with eventually diagnosed MPE (close to two-thirds lung cancer) resulted in a yield of approximately 90% irrespective of the presence or absence of pleural thickening (22). Either a blunt or cutting needle was used in the presence of pleural thickening, and only a blunt needle in its absence. When CT assistance for blunt needle biopsy was compared to US assistance for cutting needle biopsy for the diagnosis of MPE (about one-quarter lung cancer), the outcome favored CT: 77% vs. 66% (23). Taken together with data from other investigators (24), these results suggest that the yield of needle biopsy of the pleura in MPE improves with image guidance rather than assistance and with a higher percentage of lung cancer relative to mesothelioma.
Thoracoscopic pleural biopsy
With the advent of video-assisted thoracoscopic surgery (VATS), surgical pleural biopsy can be performed with less morbidity compared to traditional open thoracotomy, but the rate of post-operative complications is not negligible (25,26). Patients undergoing this procedure must be able to tolerate general anesthesia with single-lung ventilation and may experience lingering post-operative pain (27). These features of VATS pleural biopsy render it unappealing in the typical MPE patient with debility and limited life expectancy. As the current “gold standard”, the yield of VATS pleural biopsy in MPE exceeds 90% (25). If available, medical thoracoscopy (MT) is an alternative to VATS that can be performed under local anesthesia with or without sedation. This procedure entails the induction of an obligatory pneumothorax, which the patient must be able to tolerate while breathing spontaneously. Forceps biopsies of the parietal pleura can be performed during MT through a rigid or semi-rigid thoracoscope. Like that of VATS, the yield of this procedure in pleural malignancy exceeds 90% (28), and major complications are rare, though even death has been reported (29). A randomized trial comparing biopsy during MT with CT-assisted blunt needle biopsy of the pleura included 80 patients with eventual pleural malignancy, 29 (36%) of whom turned out to have metastatic lung cancer (30). Sensitivity of MT was numerically but not statistically superior to that of needle biopsy (95% vs. 87%) for all types of MPE. In cases with lung cancer as the primary tumor, yields were nearly identical: 14/15 (93%) for needle biopsy and 14/14 (100%) for MT. Complication rates were low and comparable for both procedures.
Management
General principles
The presence of an MPE in a patient with lung cancer is designated as intrathoracic metastatic disease (M1a) in the most recent 8th edition of the International Association for the Study of Lung Cancer Tumor, Node, Metastasis (TNM) classification system (31). The M1a designation translates to stage IV disease, the fundamental treatment for which is palliative platinum-based doublet chemotherapy. Traditional chemotherapeutic agents are limited in their ability to reliably control the accumulation of pleural fluid with (32) or without (33) the addition of VEGF inhibitor bevacizumab and whether administered intravenously or intrapleurally (34). The ever-expanding role of targeted therapy aimed at driver mutations and the recent advent of checkpoint inhibition for stage IV lung cancer may eventually change the landscape of systemic MPE therapeutics, but at present the mainstay of management remains local procedural interventions in the pleural space (35). The primary treatment objective in these patients with incurable lung cancer is symptom relief and thereby quality of life improvement.
Thoracentesis
Therapeutic thoracentesis must be performed upon initial encounter with a suspected symptomatic MPE. Besides characterizing the pleural fluid and collecting a cytology specimen, this procedure allows determination of the impact of fluid removal on symptoms and assessment of the compressed lung’s ability to re-expand. Information thus obtained is vital for planning subsequent steps. For patients with extremely short life expectancy, therapeutic thoracentesis can be viewed as definitive management if the need for multiple drainages before death is considered unlikely. Because the majority of patients with MPE will experience recurrence before death—often within two weeks of initial thoracentesis—the more durable strategies discussed below constitute superior options (36).
Pleurodesis
The principle behind pleurodesis is that the pleural space is not essential to life and thus can be sealed with the resultant benefit of cessation of fluid entry. The classical mechanism envisions an intense pleuritis triggered by a deliberate insult with consequent symphysis of the pleural membranes, which are thereby expected to fuse together completely and permanently by forming adhesions. Pleurodesis can be considered complete if fluid does not reaccumulate at all for the lifetime of the patient, partial if there is clinically insignificant reaccumulation, and failed if a repeat pleural procedure becomes necessary (37). The effectiveness of pleurodesis can wane over time, so the success rate varies as a function of the time point at which it is assessed. The mechanism underlying pleurodesis is predicated on the ability of the visceral and parietal pleural surfaces to achieve apposition. It follows from this assumption that pleurodesis will be infeasible if the visceral pleura cannot reach the parietal pleura due to failure of lung re-expansion as happens in cases of malignant lung entrapment or endobronchial obstruction.
A mechanical means of accomplishing pleurodesis is pleural abrasion, which requires an operative approach. Many different chemical pleurodesis agents have been used in clinical practice, and their delivery is possible thoracoscopically or via tube thoracostomy. In the English-speaking world, talc is the most commonly used pleural sclerosant (38). It is also the most effective agent and the most extensively studied (39). In the TIME1 randomized controlled trial (RCT) in which 294 patients with MPE underwent talc pleurodesis and in which pleurodesis failure was defined as the need for repeat pleural intervention, at 3 months close to 80% of patients remained successfully pleurodesed (40). An area of ongoing controversy is whether thoracoscopic talc poudrage is superior to its bedside administration as slurry. The largest RCT completed so far to answer this question did not demonstrate a significant difference in pleurodesis success (defined radiographically) in the entire study population at 30 days, but notably a post-hoc subgroup analysis did detect an advantage to poudrage in patients with lung or breast cancer (41). Pooled analysis of the available data included in a recent clinical practice guideline yielded comparable efficacy and safety results between the two methods of talc delivery (42). Safety concerns regarding talc pleurodesis stem from occurrences of the acute respiratory distress syndrome (ARDS), believed to be the result of extrapleural inflammation induced by small talc particles that manage to escape into the systemic circulation (43). Lending support to this theory is the observation that ARDS cases were reported in a US study population that received ungraded talc (44) (i.e., containing small particles) but were not seen in a European cohort that received graded large-particle talc (45). For this reason, only the latter talc preparation is currently recommended and available for clinical use.
The second most popular pleural sclerosant is doxycycline, a tetracycline derivative that is somewhat less effective than talc (38,46). Its instillation into the pleural space causes intense inflammation and is therefore notoriously painful. Both topical and systemic analgesia is typically administered when doxycycline is used in an awake patient. Traditionally, opiate analgesics have been the preferred option for pain control while non-steroidal anti-inflammatory drugs (NSAIDs) have been avoided for fear of interference with pleurodesis. Notably, the TIME1 trial investigators compared the efficacy of talc pleurodesis in subjects with MPE receiving opiates for pain vs. those receiving NSAIDs and found pleurodesis outcome to be non-inferior with the latter. Doxycycline has not been associated with systemic toxicity such as ARDS.
Indwelling pleural catheter (IPC)
An alternative to pleurodesis for MPE that has gained popularity is the IPC designed to allow outpatient self-drainage (Figure 1). The catheter can be inserted on an ambulatory basis and is tunneled through subcutaneous tissue in order to reduce the likelihood of dislodgement and infection. The patient performs home-based pleural fluid drainage usually with the assistance of a family member or nurse either on a fixed schedule or as needed for symptom relief. A properly functioning IPC is virtually guaranteed to provide effective fluid control (47). Approximately 20% of patients experience IPC-related complications, however, the most common being infection and catheter malfunction (47-49). Many fewer patients—about 7%—require catheter removal due to complications, which tend to be minor (36,49). For example, unlike parapneumonic pleural space infection, IPC-related infection is rarely fatal, and international experience suggests that it can often be successfully treated while preserving the catheter (50). Failure of IPC drainage due to multiloculation of fluid typically leads to an attempt at fibrinolysis through the catheter, though a recent RCT showed that intrapleural urokinase does not alleviate dyspnea in symptomatic MPE patients who develop this problem (51). It has been long recognized that some percentage of MPE cases managed with an IPC will undergo eventual catheter removal when fluid formation ceases due to so-called “spontaneous pleurodesis”, presumed to occur as a result of pleural irritation by the catheter itself (52). Estimates from observational data and RCTs not designed to study this outcome specifically place the rate of spontaneous pleurodesis at roughly 50% (36,47,49,53). Information from dedicated randomized trials reveals variability depending on time of assessment and drainage protocol. One RCT reported a spontaneous pleurodesis rate of 23% at 35 days and 27% at 70 days (54). An RCT studying drainage frequency as the primary variable showed a significantly higher spontaneous pleurodesis rate at 3 months in those who drained daily (47%) compared to those who drained on alternate days (24%) (55). From available data, it is possible to conclude that an approximately 50% spontaneous pleurodesis rate can be achieved with an IPC placed for MPE, but that number is reached after about 3 months of daily drainage. Importantly, the benefit of daily drainage appears to be limited to increasing the likelihood of spontaneous pleurodesis because a symptom-based regimen has been shown to be equally effective in controlling dyspnea (56).
Selection of management strategy
Certain circumstances can a priori favor the choice of either pleurodesis or IPC for the management of MPE in a given patient. For example, the presence of a pleural drain at time of decision-making could favor bedside pleurodesis. If metastatic pleural malignancy is established during thoracoscopy, talc could be insufflated for pleurodesis during the same procedure. Contact between visceral and parietal pleural surfaces is a necessary condition for successful pleurodesis. Therefore, after initial drainage of an MPE, the ability of the underlying lung to re-expand is assessed. Failure of lung re-expansion signifies either entrapment due to malignant pleural restriction or the presence of an endobronchial lesion preventing aeration. When such a situation is encountered, IPC becomes the default option if pleural fluid is contributing to symptoms (42). On the other hand, the absence of adequate family or professional support to assist with the drainage procedure in the patient’s residence is a practical argument against the placement of an IPC. Notwithstanding these special considerations, most MPE patients are eligible for both interventions (57). Thoracoscopic pleurodesis, especially via VATS, can be prohibitively invasive for the frail, debilitated MPE patient, which is a common phenotype.
The first direct prospective comparison between IPC and pleurodesis for MPE was a trial of inpatients predominantly with lung cancer using doxycycline administration via tube thoracostomy (48). The most striking finding of this early study was the significant reduction in median hospital days in the IPC arm (1.0) vs. the pleurodesis arm (6.5). Of the patients with an initial successful doxycycline pleurodesis, 79% maintained durable fluid control throughout follow up, and in the IPC group 89% did not require subsequent medical attention for drainage problems. The MIST2 trial, a UK-based RCT, randomized hospitalized participants with MPE to IPC or talc slurry pleurodesis and assessed the patient-centered outcome of dyspnea at 7 weeks as its primary endpoint (49). Both interventions significantly improved dyspnea by that time point with no difference observed between them. At 6 months, however, the IPC group experienced less dyspnea than the pleurodesis group with a difference that was both statistically and clinically significant but one that did not translate into an accompanying difference in quality of life. Complications were overall more common in the IPC arm, but only minor complications were significantly increased. As with doxycycline, talc slurry pleurodesis resulted in a significantly shorter index hospitalization: median 0 vs. 4 days. A subsequent Australasian trial also confirmed this finding and further determined that IPCs reduce by 2 days an MPE patient’s total inpatient stay in the remainder of life as compared to talc slurry pleurodesis (58). Meta-analysis data bear out another advantage of IPC over talc pleurodesis: reduced requirement for repeat pleural procedures (59). A cost-effectiveness comparison between talc pleurodesis and IPC most strongly favors the latter if professional drainage assistance is not required and the patient survival is shorter than 14 weeks, resulting in cost containment vis-à-vis nursing care and catheter supplies, respectively (60).
An intriguing answer to the pleurodesis vs. IPC dilemma is the option of combining these modalities. Its feasibility was first proposed in a pilot study wherein patients with established MPE underwent, in the same procedure, thoracoscopic talc poudrage followed by IPC placement for the possibility of pleurodesis failure, which then allowed rapid hospital discharge within a median of 1.8 days (61). Over 90% of the patients achieved successful pleurodesis and so underwent IPC removal after a median of 7 days. This strategy requires a more invasive procedure than bedside pleurodesis, and its cost-effectiveness has been questioned (62). Transferring the concept of IPC as insurance against pleurodesis failure to the bedside takes advantage of the ability of the IPC to serve as a conduit for instillation of sclerosing agents and assumes that MPE patients place high value on becoming catheter-free. The hope would be that chemical pleurodesis through the IPC could increase the intrinsic spontaneous pleurodesis rate of the IPC itself and shorten the time to its development. Initial fluid control with the IPC could also allow rapid transition to the outpatient setting while awaiting chemical pleurodesis. Concerns with the administration of talc slurry in this fashion would be occlusion of a long-term catheter and the combination of two factors (IPC and talc) known to promote fluid multiloculation. The results of such an approach were reported in a series of 24 MPE patients, majority with lung cancer, who received talc slurry via a recently inserted IPC—all but two underwent outpatient pleurodesis (63). Pleurodesis was successful in 22 of the 24 cases (92%) and all 22 had their IPC removed 14 days after receiving talc. No instances of catheter blockage or fluid multiloculation were encountered. Subsequently, this combined strategy was subjected to a multi-center trial in the UK that randomized 154 IPC recipients with MPE to outpatient talc pleurodesis or placebo if after 10 days they achieved adequate pleural apposition (54). The majority of participants had lung cancer. The primary outcome measure, successful pleurodesis by day 35, was achieved by a significantly greater percentage of patients who received talc compared to those who did not: 43% vs. 23%. The difference in favor of talc remained significant at 70 days (51% vs. 27%), which was a secondary endpoint. There was no significant difference in the rate of adverse events, including drainage problems due to catheter occlusion or fluid multiloculation.
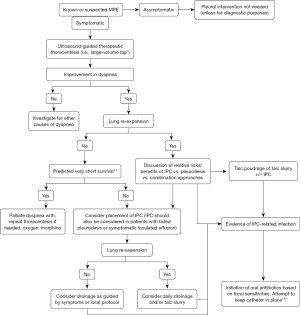
In summary, the unresolved question of superiority between pleurodesis and IPC for MPE management may eventually be answered by combining the two modalities rather than by elevating one over the other. If no compelling indications or contraindications for either intervention pertain and the combined strategy is not an option, the choice becomes a product of patient preference and clinical pragmatism. Recent expert guidelines advocate the same approach, which is incorporated into a proposed MPE management algorithm (Figure 2) (42).
Prognosis and outcomes
Regardless of its size, the presence of MPE in lung cancer is an independent adverse prognostic indicator (4). According to the large multinational database used to derive the 8th edition of the TNM staging system for lung cancer, the median survival of lung cancer patients with pleural and/or pericardial effusion is approximately 11 months (31). Individual center experience suggests that specific institutions are observing far worse survival closer to 5 months (4). Combined data from MPE cohorts in the UK, Australia, and the Netherlands paints an even bleaker picture for lung cancer: median survival of less than 11 weeks (2). Complicating MPE management decisions based on anticipated survival is the great patient to patient heterogeneity, especially in the modern era of targeted oncological therapy. To help address this problem, the so-called “LENT” score has been derived and validated with higher scores corresponding to shorter survival (2). A particular MPE patient’s LENT score increases with higher pleural fluid lactate dehydrogenase level (L), worse Eastern Oncology Cooperative Group performance status (E), higher pleural fluid neutrophil to lymphocyte ratio (N), and higher risk primary tumors (T). It is worth noting that among the commonest cell types encountered in metastatic pleural malignancy (i.e., lung, breast, gynecological, and hematological), only lung cancer belongs to the highest risk primary tumor category for LENT calculation, automatically resulting in at least a moderately elevated score. Those with moderate scores survived a median of about 4 months, whereas those with high scores had a median survival of just 44 days.
Conclusions
Despite overall progress in the survival of stage IV lung cancer patients in recent times, the occurrence of an MPE as a complication of lung cancer still portends a very poor prognosis in most cases. Confirmation of the malignant nature of a pleural effusion in a lung cancer patient should be pursued if it will have an impact on prognosis or management. The initial thoracentesis is an ideal opportunity to both establish the diagnosis of MPE and assess the contribution of fluid to respiratory symptoms if any are present. The diagnostic potential of thoracentesis is limited by suboptimal sensitivity of pleural fluid cytology, especially in certain lung cancer sub-types, so occasionally more invasive sampling is required, and the next procedure of choice depends on patient factors and availability. The primary objective of the management of MPE due to lung cancer, as with any MPE, is alleviation of discomfort caused by the effusion. Typically, lasting palliation is intricately connected to durable fluid control. Currently available means of achieving fluid control are pleurodesis and IPC placement. The former, if successful, results in fewer downstream complications but prolongs the inpatient days. About one-third to one-half of MPE patients who are managed with an IPC eventually develops a spontaneous pleurodesis, which allows catheter removal. Talc administration through a previously placed IPC has been shown to increase the likelihood of this desirable outcome, thereby lending credence to a bimodality approach. Other means of promoting this phenomenon, such as coating the IPC with a pleural sclerosant, are in the investigative phase. Anticipated patient survival time is often a factor in MPE management decisions. Prediction of this in individual lung cancer MPE patients is difficult but can be facilitated by calculating the LENT prognostic score.
Acknowledgments
None
Footnote
Conflicts of Interest: NM Rahman served as a consultant and received research support from Rocket Medical, a manufacturer of indwelling pleural catheters. O Epelbaum has no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts and Figures 2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf
- Clive AO, Kahan BC, Hooper CE. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol 2012;7:1485-9. [Crossref] [PubMed]
- Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival in lung cancer patients with pleural effusions. Respirology 2015;20:654-9. [Crossref] [PubMed]
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002;20:1545-58. [Crossref] [PubMed]
- Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. [Crossref] [PubMed]
- Porcel JM, Pardina M, Bielsa S, et al. Derivation and validation of a CT scan scoring system for discriminating malignant from benign pleural effusions. Chest 2015;147:513-9. [Crossref] [PubMed]
- Hallifax RJ, Haris M, Corcoran JP, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015;70:192-3. [Crossref] [PubMed]
- Sun Y, Yu H, Ma J, et al. The role of 18F-FDG PET/CT integrated imaging in distinguishing malignant from benign pleural effusion. PLoS One 2016;11:e0161764. [Crossref] [PubMed]
- Porcel JM, Hernández P, Martínez-Alonso M, et al. Accuracy of fluorodeoxyglucose –PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest 2015;147:502-12. [Crossref] [PubMed]
- Ferrer J, Roldán J, Teixidor J, et al. Predictors of pleural malignancy in patients with pleural effusion undergoing thoracoscopy. Chest 2005;127:1017-22. [Crossref] [PubMed]
- Sahn SA, Good JT Jr. Pleural fluid pH in malignant effusions. Diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988;108:345-9. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 thoracenteses. Arch Bronconeumol 2014;50:161-5. [PubMed]
- Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J 2018.52. [PubMed]
- Chhieng DC, Ko EC, Yee HT, et al. Malignant pleural effusions due to small-cell lung carcinoma: a cytologic and immunocytochemical study. Diagn Cytopathol 2001;25:356-60. [Crossref] [PubMed]
- Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis 2018;12:1753466618808660. [Crossref] [PubMed]
- Porcel JM, Civit C, Esquerda A, et al. Utility of CEA and CA 15-3 measurements in non-purulent pleural exudates in the diagnosis of malignancy: a single center experience. Arch Bronconeumol 2017;53:427-31. [PubMed]
- Fiorelli A, Vicidomini G, Di Domenico M, et al. Vascular endothelial growth factor in pleural fluid for differential diagnosis of benign and malignant origin and its clinical applications. Interact Cardiovasc Thorac Surg 2011;12:420-4. [Crossref] [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [Crossref] [PubMed]
- Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 1991;4:320-4. [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomized controlled trial. Lancet 2003;361:1326-30. [Crossref] [PubMed]
- Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015;70:995-7. [Crossref] [PubMed]
- Metintas M, Yildirim H, Kaya T, et al. CT Scan-Guided Abrams' Needle Pleural Biopsy versus Ultrasound-Assisted Cutting Needle Pleural Biopsy for Diagnosis in Patients with Pleural Effusion: A Randomized, Controlled Trial. Respiration 2016;91:156-63. [Crossref] [PubMed]
- Hallifax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014;146:1001-6. [Crossref] [PubMed]
- Harris RJ, Kavuru MS, Mehta AC, et al. The impact of thoracoscopy on the management of pleural disease. Chest 1995;107:845-52. [Crossref] [PubMed]
- de Groot M, Walther G. Thoracoscopy in undiagnosed pleural effusions. S Afr Med J 1998;88:706-11. [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [Crossref] [PubMed]
- Rozman A, Camlek L, Kern I, et al. Semirigid thoracoscopy: an effective method for diagnosing pleural malignancies. Radiol Oncol 2014;48:67-71. [Crossref] [PubMed]
- Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010;137:1362-8. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2015;10:1515-22.
- Tao H, Meng Q, Li M, et al. Outcomes of bevacizumab combined with chemotherapy in lung adenocarcinoma-induced malignant pleural effusion. Thorac Cancer 2018;9:298-304. [Crossref] [PubMed]
- Fujita A, Takabatake H, Tagaki S, et al. Combination chemotherapy in patients with malignant pleural effusions from non-small cell lung cancer: cisplatin, ifosfamide, and irinotecan with recombinant human granulocyte colony-stimulating factor support. Chest 2001;119:340-3. [Crossref] [PubMed]
- Figlin R, Mendoza E, Piantadosi S, et al. Intrapleural chemotherapy without pleurodesis for malignant pleural effusions. LCSG Trial 861. Chest 1994;106:363S-6S. [PubMed]
- Fortin M, Taghizadeh N, Tremblay A. Procedures performed during hospitalizations for malignant pleural effusions: Data from the 2012 National Inpatient Sample. Respiration 2018;95:228-34. [Crossref] [PubMed]
- Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest 2018;153:438-52. [Crossref] [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [Crossref] [PubMed]
- Lee YC, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest 2003;124:2229-38. [Crossref] [PubMed]
- Clive AO, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016;5:CD010529. [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA 2015;314:2641-53. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Maskell NA, Lee YC, Gleeson FV, et al. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med 2004;170:377-82. [Crossref] [PubMed]
- Gonzalez AV, Bezwada V, Beamis JF, et al. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010;137:1375-81. [Crossref] [PubMed]
- Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet (London, England) 2007;369:1535-9. [Crossref] [PubMed]
- Xia H, Wang XJ, Zhou Q, et al. Efficacy and safety of talc pleurodesis for malignant pleural effusions: a meta-analysis. PLoS One 2014;9:e87060. [Crossref] [PubMed]
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012;142:394-400. [Crossref] [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013;144:1597-602. [Crossref] [PubMed]
- Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med 2018;197:502-8. [Crossref] [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunneled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. New Engl J Med 2018;378:1313-22. [Crossref] [PubMed]
- Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. The ASAP Trial. Am J Respir Crit Care Med 2017;195:1050-7. [Crossref] [PubMed]
- Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med 2018;6:671-80. [Crossref] [PubMed]
- Fysh ETH, Bielsa S, Budgeon CA, et al. Predictors of clinical use of pleurodesis and/or indwelling pleural catheter therapy for malignant pleural effusion. Chest 2015;147:1629-34. [Crossref] [PubMed]
- Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA 2017;318:1903-12. [Crossref] [PubMed]
- Iyer NP, Reddy CB, Wahidi MM, et al. Indwelling pleural catheter versus pleurodesis for malignant pleural effusions. A systematic review and meta-analysis. Ann Am Thorac Soc 2019;16:124-31. [Crossref] [PubMed]
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017;22:764-70. [Crossref] [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [Crossref] [PubMed]
- Shafiq M, Frick KD, Lee H, et al. Management of malignant pleural effusion: a cost-utility analysis. J Bronchology Interv Pulmonol 2015;22:215-25. [Crossref] [PubMed]
- Ahmed L, Ip H, Rao D, et al. Talc pleurodesis through indwelling pleural catheters for malignant pleural effusions: retrospective case series of a novel clinical pathway. Chest 2014;146:e190-4. [Crossref] [PubMed]