Effects of sub-chronic, low-dose cadmium exposure on kidney damage and potential mechanisms
Introduction
Cadmium (Cd) is a non-essential transition metal (1) which is released into the environment by various human activities, such as mining, smelting, and refining (2). In non-occupational population, the harm of Cd to humans is mainly due to chronic exposure to low dose Cd, especially in the soil and water, because it may be taken up by crops and aquatic organisms and finally accumulated in the human body via the food chain (3-5). Thus, Cd exposure poses a major concern for public health because of its cumulative toxicity and Cd toxicity, which has been extensively studied in the past decades (6,7).
The kidney is one of the target organs of chronic Cd toxicity, and Cd exposure may cause a generalized kidney dysfunction. However, there are still controversies on the Cd-induced kidney injury and the potential mechanisms. Gao et al. (1) found polyuria was a typical feature of chronic Cd exposure related toxicity, but other studies indicated urine secretion reduced after Cd exposure (8,9). Several mechanisms have been proposed to explain the Cd related damage to organs. It has been found that the toxicity of long-term, low-dose Cd exposure is related to cell apoptosis (10), autophagy (11,12), necrosis (13), destruction of cell-cell junctions (14), and disordered cell signaling pathways (15). There is evidence (16,17) showing that Cd may initially cause the imbalance of some ions (especially Na+, K+ and Ca2+), which subsequently cause further damage to the kidney.
Since the chronic Cd exposure related renal injury and the exact mechanisms are not yet clear, this study was conducted to investigate the characteristics of renal injury after chronic, low-dose Cd exposure and the potential mechanisms were explored.
Methods
Reagents and instrument
Cd chloride (Cdcl2ˑ2.5H2O), DHE, MitoSOX, Protease Inhibitor Cocktail and Hoechst 33528 (Sigma-Aldrich Co. Ltd; St. Louis, MO, USA), bovine serum albumin (BSA) (Boehringer, USA), BCA protein test kit (Thermo Company), anti-SOD1 and anti-SOD2 antibodies (Pharmingen; San Diego, CA, USA), rabbit anti-CAT antibody (protein Tech company), rabbit Bcl-2 (Cell Signal Technology company), rabbit anti-Bax antibody (Santa Cruz Biotech Co., Ltd) and analytically pure chemicals were used in the present study. Hitachi 7020 automatic biochemical detector (Hitachi, Japan), full wave microplate reader (TECAN), positive fluorescence microscope, laser confocal microscopes (Olympus company), mini-protean Tetra electrophoresis & Blots, protein semi-dry transfer device and Gel imaging system (Bio-Rad) were used in the present study. Transmission electron microscopy (TEM) was conducted in the Key Laboratory of Dental Hospital of The Air Force Medical University.
Animal treatments
This study was approved by the Ethics Committee for Animal Experiments in the Air Force Medical University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. A total of 40 male adult Sprague-Dawley (SD) rats weighing 250–350 g were purchased from the Experimental Animal Center of Air Force Medical University. Animals were housed for 5 days and then randomly divided into four groups: control group (no Cd exposure), low-dose Cd group (1 mg/kg CdCl2); moderate-dose Cd group (2.5 mg/kg CdCl2) and high-dose Cd group (5 mg/kg CdCl2). Animals were given ad libitum access to water and food during the experiment and housed in an environment at 24±2 °C with the humidity of 50%±10% and 2 h/12 h light-dark cycle.
CdCl2 was intragastrically administered once daily for consecutive 60 days. After CdCl2 treatment, animals were intraperitoneally anesthetized with 2% pentobarbital sodium (Syntec, USA) at 100 mg/kg, and blood was collected from the abdominal aorta. The kidneys and liver were collected and their wet weight was obtained. The ratio of kidney weight/body weight and the liver weight/body weight were then calculated. The left renal cortex was divided into three parts: one was stored in liquid nitrogen, one was fixed in 4% formaldehyde for histological examination and one was fixed in 2% glutaraldehyde for TEM. The right kidney was stored at −80 °C for the following experiments.
Detection of kidney function and related indicators
The blood was collected and centrifuged for 10 min at 3,500 r/min. The serum was harvested and the concentrations of BUN, SCr, β2-MG, Cd2+, Fe2+ and Ca2+ were detected by using Hitachi 7020 automatic biochemical analyzer.
Detection of serum antioxidant enzymes and oxidative products
The levels of MDA, GSH, SOD and CAT in the serum were detected according to the manufacturer’s instructions using corresponding assay kits (Jiancheng Bioengineering Institute; Nanjing, China).
Histopathological examination
Kidneys were fixed in 4% formaldehyde for 48 h, embedded in paraffin and sectioned (thickness: 5 µm). Sections were stained with hematoxylin-eosin (H&E), followed by microscopy.
Examination of mitochondrial ultrastructure
The renal cortex was separated and cut into 1-mm slices which were then fixed in 2% glutaraldehyde and sectioned for electron microscopy. The mitochondrial ultrastructure in the renal proximal tubular epithelial cells was observed under a transmission electron microscope.
Detection of ROS in the renal cortex
The fluorescence probe DHE was used to detect the level of ROS in the renal cortex, and the probe MitoSOX to detect ROS in the mitochondria. In brief, 10-µm renal cortex sections were placed in the wet box; 10-µM DHE and 5 mM MitoSOX were diluted with PBS to 1:1,000 and 1:500, respectively, and then mixed with 1:500 Hoechst; the mixture (150 µL) was added onto each section, followed by incubation in dark for 40 min at 37 °C. After mounting with Glycerine, sections were washed thrice with PBS, and then observed under a laser scanning confocal microscope. The level of ROS was determined after representative photographs were obtained.
Western blotting
The expression of target proteins in the renal tissues was detected by Western blotting. In brief, 100 mg of renal cortex was homogenized in 1 mL of lysis buffer (1 mL RIPA + 20 µL Na3VO4 + 5 µL cocktail) for 30 min at 4 °C. The supernatant was collected after centrifugation for 20 min at 10,000 r/min. Then, 20 µL of supernatant was used to quantify the protein concentration with BCA method. The remaining protein solution was mixed with 2× SDS of equal volume and 5 µL of DTT, followed by boiling at 100 °C for 5 min. Membranes were incubated with primary antibodies at 1:5,000 overnight at 4 °C, followed by washing thrice; then membranes were incubated with secondary antibodies at 37 °C for 40 min. After washing 4 times with TBST (15 min for each), the expression of target proteins was detected after visualization with ELC reagents.
Statistical analysis
Statistical analysis was performed with SPSS software (version 21.0) and data are presented as mean ± standard deviations (mean ± SD). The normality and homogeneity of these data were tested and the differences among groups were assessed with one-way analysis of variance (ANOVA) followed by LSD t-test, or non-parametric test. A value of P<0.05 was considered statistically significant.
Results
General conditions of animals after chronic Cd exposure
During the entire experiment, the food intake of animals in the control group was normal. However, the rats after chronic Cd exposure displayed loss of appetite, rough fur and irritable mood, and the higher the dose of CdCl2, the more evident these changes were.
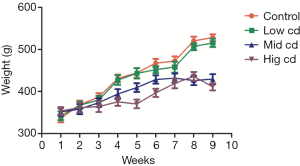
Body weight after chronic Cd exposure
From the third week, the body weight of rats in moderate-dose and high-dose groups decreased significantly as compared to the control group (P<0.05), and this continued until the end of experiment (Figure 1).
Liver weight /body weight ratio, kidney weight /body weight ratio, water intake and urine volume after chronic Cd exposure
The water intake and 24-hour urine volume were determined before sacrifice. Results showed the liver weight to body weight ratio increased markedly, and the 24-hour urine volume and water intake decreased significantly in the moderate-dose and high-dose groups as compare to the control group (P<0.05) (Table 1).

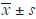 )
) Full table
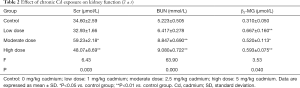
Renal function after chronic Cd exposure
The kidney function was evaluated by the serum BUN, SCr and β2-MG. As shown in Table 2, the serum BUN, SCr and β2-MG increased significantly in a Cd dose dependent manner (P<0.05) (Table 2).
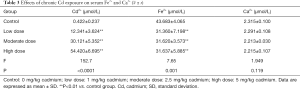
Serum Fe2+ and Ca2+ concentrations after chronic Cd exposure
As shown in Table 3, the Cd2+ concentration increased significantly but the Fe2+ concentration decreased markedly in a Cd-dose dependent manner (P<0.05). However, there was no significant difference in the serum Ca2+ concentration between groups (P>0.05).
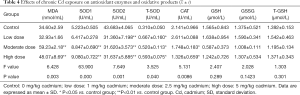
Serum antioxidant enzymes and oxidative products after chronic Cd exposure
As shown in Table 4, the serum contents of MDA and SOD1 increased significantly, but those of SOD2 and CAT decreased markedly as compared to the control group (P<0.05). However, there were no significant differences among four groups in the serum contents of reduced glutathione and oxidized glutathione.
Histopathology after chronic Cd exposure
Representative figures of H&E staining are shown in Figure 2. In the control group, the microscopic structure of the kidney was complete, the glomeruli were regular, the capillary network was clear, the renal tubules had clear outline, the surface of the brush was clear, and the epithelial cells were arranged regularly. However, the pathological changes of the kidney deteriorated with the increase in Cd dose. In low-dose group, the glomerular structure was intact, the renal tubules had slightly swelling and the interstitium showed hyperemia. In moderate-dose group, the glomerular and renal tubules displayed significantly swelling, the structure was damaged severely, the number of cells increased, the cells were arranged irregularly, the lumens were narrow, the epithelial cells were necrotic, the interstitium had hyperemia and infiltration of inflammatory cells. In high-dose group, the glomeruli were swelling, the capillary network was cracked, renal tubules were extensively lesioned, the epithelial cells became necrotic, a large number of cells accumulated in the lumen and were positive for eosin, renal interstitial congestion was more serious, and more inflammatory cells infiltrated into the interstitium.
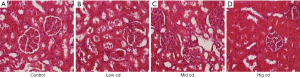
Mitochondrial ultrastructure after chronic Cd exposure
The mitochondria ultrastructure is shown in Figure 3. Chronic Cd exposure mainly caused mitochondrial swelling, deformation, vacuolar degeneration, and mitochondrial ridge abnormality. The degree of injury gradually increased with increase in Cd dose.
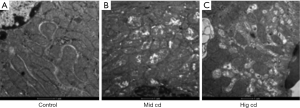
Level of ROS in the renal cortex and mitochondria after chronic Cd exposure
The level of ROS in the renal cortex and mitochondria was detected by DHE staining and MitoSOX staining, respectively. As shown in Figure 4, ROS level in three Cd exposure groups increased significantly, especially in the mediate-dose group.
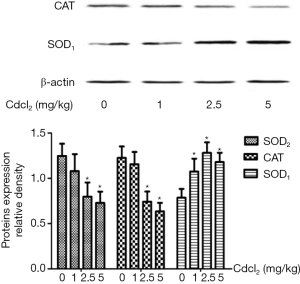
Expression of antioxidant enzymes in the kidney after chronic Cd exposure
As shown in Figure 5, when compared with the control group, the expression of SOD2 in the mitochondria and CAT in the cytoplasm reduced significantly, but the expression of SOD1 in the cytoplasm increased markedly in the Cd exposure groups (P<0.05). These changes were dependent on the dose of Cd.
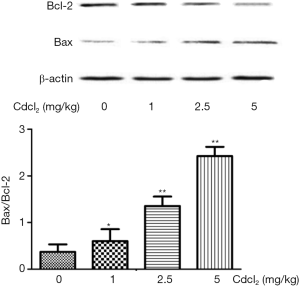
Expression of apoptosis related proteins in the kidney after chronic Cd exposure
As compared to the control group, the Bcl-2 expression decreased, but the Bax expression and Bax/Bcl-2 ratio increased significantly in a Cd-dose dependent manner (P<0.05) (Figure 6).
Discussion
Every year, thousands of tons of Cd-containing pollutants are discarded into our environment, which poses an increasing health risk to our food and drinking water (18). It has been accepted Cd can accumulate in many organs of humans, including the liver, kidney, pancreas and testis, adversely affecting the functions of these organs. Among them, kidney is recognized as a major target organ of Cd-induced toxicity (19).
The present study investigated the renal injury after sub-chronic, low-dose Cd exposure. The body weight, the ratio of liver weight to body weight and the ratio of kidney weight to body weight are important non-specific indicators in animal experiments (20) and can comprehensively reflect the poisoning on the animals. Therefore, some investigators pay attention to these indicators in the animal toxicology. Our study showed, from the third week, the body weight in the moderate-dose and high-dose exposure groups decreased significantly; the liver weight/body weight ratio in high-dose group increased markedly but the kidney weight/body weight ratio was comparable to that in the control group. These were inconsistent with those in the study of Wang et al. (21), who found a significant difference in the kidney weight to body weight ratio but not in the body weight throughout the experiment. This may be ascribed to the difference in animal species, or body weight. In the study of Wang et al., Wistar rats weighing 180–200 g were used and these animals were younger than the animals in our study. Our study aimed to investigate the response of middle-aged SD rats to Cd exposure, because the middle age is in a danger stage for all types of kidney diseases. The ratio of liver weight to body weight increased in Cd exposure groups. This might be ascribed to the hepatic toxicity of Cd2+ in acute phase after it was absorbed into the blood and thereafter transported to the liver, and compensatory hepatomegaly was present after chronic Cd exposure. The kidney is relatively small and its volume usually varies slightly because it is a solid organ. Thus, in our study, the ratio of kidney weight to body weight remained unchanged although the function and cellular structure were significantly damaged.
In our study, the 24-hour urine volume and water intake in moderate-dose and high-dose Cd groups consistently decreased, but Wallin et al. (22) found that urine secretion increased after Cd exposure. This might be related to the difference in the exposure time and the way by which exposure was performed. In our study, sub-chronic exposure was employed. We speculated that in early and middle stages of Cd exposure, glomerular filtration was limited, the filtration membrane was damaged, the renal artery contracted, the capillary became narrow, and the filtration rate decreased, leading to oliguria. In addition, Cd2+ may affect the body metabolism of rats, and thus rats drink less, leading to the reduced urine secretion. Cd2+ induced metabolic disorders have been confirmed in many studies (1). The general conditions (such as dry hair and irritability) of rats after chronic Cd exposure were consistent with previously reported (23).
In present study, BUN and Scr were used to evaluate the glomerular function and β2-MG to assess the renal tubular damage (24). Elevated BUN indicates substantial renal lesions (25) (such as glomerulonephritis). Scr mainly reflects the glomerular filtration, and it increases when the glomerular filtration reduces (26). Although Scr is able to accurately reflect the renal parenchymal damage, but it is not a sensitive indicator. As shown in our results, serum BUN, SCr and β2-MG increased in a Cd dose dependent manner. Results indicated Cd seriously damaged the glomeruli after 60-day exposure. In the present study, serum β2-MG was at a low level, and it in moderate-dose and high-dose Cd groups significantly increased, indicating that the renal tubular injury was present and it occurred earlier than glomerular damage.
Recently, Prozialeck et al. (27) found that Cd2+ could enter the renal tubular cells through a variety of channels and transporters for metal ions such as Ca2+, Fe2+, and Zn2+. Findings from human and animal studies have revealed (28) that deficiencies in these metal ions, imbalance of local metal ions, and abnormality in the metabolism of these metal ions are potential causes of kidney dysfunction. For example, Cd2+ can directly compete with Ca2+ to inhibit the Ca2+ transportation in cells or Cd2+ directly influences the kidney function to decrease 1,25(OH)2D3 synthesis and indirectly affects Ca2+ metabolism. Our results showed that the Fe2+ concentration decreased with the increase in Cd2+ dose, but there was no difference in the Ca2+ concentration among groups. Thus, Cd2+ may inhibit the Fe2+ absorption or competitively antagonize the transportation of Fe2+ into the blood during the sub-chronic, low-dose Cd exposure. Whether this affects the activity of Fe2+-related enzymes, such as CAT, to further influence the redox homeostasis, resulting in renal dysfunction is still unclear. Thus, the serum content of antioxidases and protein expression of them in the renal cortex were further detected.
Our results showed the expression of SOD1 in the cytoplasm increased, but that of SOD2 in the mitochondria decreased significantly in the serum and renal cortex as compared to the control group. These suggest that Cd2+ induced nephrotoxicity may cause redox imbalance, leading to oxidative stress and mitochondrial damage. CAT is important for H2O2 scavenging (29) and contains four Fe atoms. Coincidentally, in our experiments, both Fe2+ and CAT reduced significantly. CAT is a conjugate enzyme with ferric porphyrin and can scavenge H2O2 with the participation of Fe atoms. Therefore, we postulate that Cd2+ can affect the absorption of Fe2+ or compete with Fe2+ to bind to CAT, leading to the decreased CAT expression, which further induces the increase in ROS, subsequent oxidative stress, and final renal injury. The CAT expression in the serum and renal cortex decreased consistently, which further proved this hypothesis. MDA is one of the most important products of membrane lipid peroxidation (30), and may affect the mitochondrial respiratory chain complex and the activity of key enzymes in the mitochondrion. In the present study, MDA significantly increased, which further indicates that Cd2+ significantly damages the mitochondria in the kidney, increasing oxidative injury. There was no significant difference in the serum GSH between control and Cd treated groups. A large number of studies have shown that Cd2+ can reduce the active of GSH (31,32), which was not observed in our study. We speculate that activity of different antioxidant enzymes will change with the prolongation of Cd2+ exposure, there is a clear time-dependent manner in the Cd2+ related antioxidant enzyme activity, but the expression of antioxidant enzymes is different from their activity.
Mitochondrion is an important organelle in cells, can generate ATP for various catabolic and anabolic processes and is also an important source of ROS that are mainly generated via the electron transport chain (33). However, mitochondria are also sensitive to ROS formed in extra-mitochondrial compartments, thereby contributing to apoptosis and aging. The mitochondrial structural damage and dysfunction may cause organ damage and systemic disease (34). Mounting evidence has shown that Cd can indirectly promote the production of ROS through metal containing anti-oxidative enzymes after the metal ion in these enzymes is replaced with Cd (35). Our results revealed the mitochondria of renal tubular epithelial cells showed swelling, mitochondria ridges disappeared, and vacuolization was present under a transmission electron microscope. Meanwhile, DHE and MitoSOX staining showed the ROS level in the kidney increased in moderate-dose and high-dose Cd groups. ROS is closely related to mitochondrial injury. Thus, our results indicate that Cd2+ causes the increase in ROS production, which leads to oxidative stress, resulting in renal mitochondrial damage.
The expression of apoptosis related proteins Bax and Bcl-2 was also detected in the renal cortex. Results showed the expression of anti-apoptotic Bcl-2 decreased, but that of pro-apoptotic Bax and the Bax/Bcl-2 ratio increased with the increase in Cd dose. This suggests that Cd exposure may cause apoptosis of renal cells in a dose-dependent manner, which is consistent with previously reported (36,37).
In conclusion, our study indicates that sub-chronic, low-dose Cd exposure may disrupt redox balance, induce apoptosis and cause renal damage. Cd2+ may decrease the content of Fe2+ in the kidney because Cd2+ inhibits the uptake of Fe2+ in the intestine, decreases the intake of Fe2+ due to weight loss, eventually inhibits the activity of Fe2+ containing CAT, and increases ROS production and lipid peroxidation, which induce oxidative stress, damage mitochondria and cause kidney dysfunction.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No: 81473010), the Shaanxi Natural Science Fund (2016JM8031), the Sha’anxi Administration of Traditional Chinese Medicine Fund (JCMS035) and the Research Project in Sha’anxi University of Chinese Medicine (2016PY13).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved the Ethics Committee for Animal Experiments in the Air Force Medical University.
References
- Gao Y, Lu Y, Huang S, et al. Identifying early urinary metabolic changes with long-term environmental exposure to cadmium by mass-spectrometry-based metabolomics. Environ Sci Technol 2014;48:6409-18. [Crossref] [PubMed]
- Mezynska M, Brzoska MM. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res Int 2018;25:3211-32. [Crossref] [PubMed]
- Arbi S, Oberholzer HM, Van Rooy MJ, et al. Effects of chronic exposure to mercury and cadmium alone and in combination on the coagulation system of Sprague-Dawley rats. Ultrastruct Pathol 2017;41:275-83. [Crossref] [PubMed]
- Cobbina SJ, Chen Y, Zhou Z, et al. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J Hazard Mater 2015;294:109-20. [Crossref] [PubMed]
- Samarghandian S, Azimi-Nezhad M, Shabestari MM, et al. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscip Toxicol 2015;8:151-4. [Crossref] [PubMed]
- Babaknejad N, Bahrami S, Moshtaghie AA, et al. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol Trace Elem Res 2018;185:106-15. [Crossref] [PubMed]
- Liu Y, Xiao W, Shinde M, et al. Cadmium favors F-actin depolymerization in rat renal mesangial cells by site-specific, disulfide-based dimerization of the CAP1 protein. Arch Toxicol 2018;92:1049-64. [Crossref] [PubMed]
- Brzoska MM. Low-level chronic exposure to cadmium enhances the risk of long bone fractures: a study on a female rat model of human lifetime exposure. J Appl Toxicol 2012;32:34-44. [Crossref] [PubMed]
- Brzoska MM, Majewska K, Kupraszewicz E. Effects of low, moderate and relatively high chronic exposure to cadmium on long bones susceptibility to fractures in male rats. Environ Toxicol Pharmacol 2010;29:235-45. [Crossref] [PubMed]
- Swiergosz R, Zakrzewska M, Sawicka-Kapusta K, et al. Accumulation of cadmium in and its effect on bank vole tissues after chronic exposure. Ecotoxicol Environ Saf 1998;41:130-6. [Crossref] [PubMed]
- Chen M, Li X, Fan R, et al. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere 2018;194:396-402. [Crossref] [PubMed]
- Fujishiro H, Liu Y, Ahmadi B, et al. Protective effect of cadmium-induced autophagy in rat renal mesangial cells. Arch Toxicol 2018;92:619-31. [Crossref] [PubMed]
- Diaz E, Perez D, Delgado Acevedo J, et al. Longitudinal survey of lead, cadmium, and copper in seagrass Syringodium filiforme from a former bombing range (Vieques, Puerto Rico). Toxicol Rep 2017;5:6-11. [Crossref] [PubMed]
- So KY, Lee BH, Oh SH. The critical role of autophagy in cadmium-induced immunosuppression regulated by endoplasmic reticulum stress-mediated calpain activation in RAW264.7 mouse monocytes. Toxicology 2018;393:15-25. [Crossref] [PubMed]
- Zhang C, Lin J, Ge J, et al. Selenium triggers Nrf2-mediated protection against cadmium-induced chicken hepatocyte autophagy and apoptosis. Toxicol In Vitro 2017;44:349-56. [Crossref] [PubMed]
- Chen BC, Wang PJ, Ho PC, et al. Nonlinear biotic ligand model for assessing alleviation effects of Ca, Mg, and K on Cd toxicity to soybean roots. Ecotoxicology 2017;26:942-55. [Crossref] [PubMed]
- Ha TT, Burwell ST, Goodwin ML, et al. Pleiotropic roles of Ca(+2)/calmodulin-dependent pathways in regulating cadmium-induced toxicity in human osteoblast-like cell lines. Toxicol Lett 2016;260:18-27. [Crossref] [PubMed]
- Yang H, Shu Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci 2015;16:1484-94. [Crossref] [PubMed]
- Cobbina SJ, Chen Y, Zhou Z, et al. Interaction of four low dose toxic metals with essential metals in brain, liver and kidneys of mice on sub-chronic exposure. Environ Toxicol Pharmacol 2015;39:280-91. [Crossref] [PubMed]
- Sanchez-Chardi A, Ribeiro CA, Nadal J. Metals in liver and kidneys and the effects of chronic exposure to pyrite mine pollution in the shrew Crocidura russula inhabiting the protected wetland of Donana. Chemosphere 2009;76:387-94. [Crossref] [PubMed]
- Wang B, Luo Q, Shao C, et al. The late and persistent pathogenic effects of cadmium at very low levels on the kidney of rats. Dose Response 2013;11:60-81. [Crossref] [PubMed]
- Wallin M, Sallsten G, Lundh T, et al. Low-level cadmium exposure and effects on kidney function. Occup Environ Med 2014;71:848-54. [Crossref] [PubMed]
- Abd-Elhakim YM, El Sharkawy NI, El Bohy KM, et al. Morphological, biochemical, and histopathological postmortem ocular indices following subchronic exposure to cadmium and/or lead in a rabbit model. Environ Sci Pollut Res Int 2018;25:6619-32. [Crossref] [PubMed]
- Ghoochani M, Rastkari N, Yunesian M, et al. What do we know about exposure of Iranians to cadmium? Findings from a systematic review. Environ Sci Pollut Res Int 2018;25:1-11. [Crossref] [PubMed]
- Cai Y, Lee J, Wang W, et al. Effect of Cd2+ on muscle type of creatine kinase: Inhibition kinetics integrating computational simulations. Int J Biol Macromol 2016;83:233-41. [Crossref] [PubMed]
- Timbrell JA. Urinary creatine as a biochemical marker of chemical induced testicular damage. Arh Hig Rada Toksikol 2000;51:295-303. [PubMed]
- Prozialeck WC, Edwards JR. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther 2012;343:2-12. [Crossref] [PubMed]
- Zhang B, Fang CD, Xu JH, et al. Depuration of Cadmium from Blue Mussel (Mytilus edulis) by Protein Hydrolysate-Fe(2+) Complex: The Role of Metallothionein. J Food Sci 2017;82:2767-73. [Crossref] [PubMed]
- Belyaeva EA, Emelyanova LV, Korotkov SM, et al. On the mechanism(s) of membrane permeability transition in liver mitochondria of lamprey, Lampetra fluviatilis L.: insights from cadmium. Biomed Res Int 2014;2014:691724. [Crossref] [PubMed]
- Li Y, Zhang S, Jiang W, et al. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ Sci Pollut Res Int 2013;20:1117-23. [Crossref] [PubMed]
- Per TS, Masood A, Khan NA. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017;68:111-24. [Crossref] [PubMed]
- Ullah H, Khan MF, Jan SU, et al. Depletion of GSH in human blood plasma and cytosolic fraction during cadmium toxicity is temperature and pH dependent. Pak J Pharm Sci 2016;29:89-95. [PubMed]
- Paesano L, Perotti A, Buschini A, et al. Markers for toxicity to HepG2 exposed to cadmium sulphide quantum dots; damage to mitochondria. Toxicology 2016;374:18-28. [Crossref] [PubMed]
- Xiang X, Wu C, Zhang BR, et al. The relationship between the length of surface ligand and effects of CdTe quantum dots on the physiological functions of isolated mitochondria. Chemosphere 2017;184:1108-16. [Crossref] [PubMed]
- Nair AR, Lee WK, Smeets K, et al. Glutathione and mitochondria determine acute defense responses and adaptive processes in cadmium-induced oxidative stress and toxicity of the kidney. Arch Toxicol 2015;89:2273-89. [Crossref] [PubMed]
- Wan N, Xu Z, Liu T, et al. Ameliorative Effects of Selenium on Cadmium-Induced Injury in the Chicken Ovary: Mechanisms of Oxidative Stress and Endoplasmic Reticulum Stress in Cadmium-Induced Apoptosis. Biol Trace Elem Res 2018;184:463-73. [Crossref] [PubMed]
- Wang XY, Yang H, Wang MG, et al. Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis 2017;8:e3099. [Crossref] [PubMed]