
Ability of serum procalcitonin to distinguish focus of infection and pathogen types in patients with bloodstream infection
Introduction
Bloodstream infection (BSI) caused by pathogenic bacteria infection is a common clinical illness which could pose life-threatening situation to patients, BSI usually appears in departments such as medicine, clinical surgery, neonatology and intensive care units (ICU). Especially, the incidence of BSI in ICU department is 30–40% (1), and the mortality rate of BSI can be up to 41.2% (2). Early diagnosis and treatment of BSI is critical to prognosis; however, rapid diagnosis is a challenge for clinical departments. It is well known that blood culture (BC) is the widely recognized gold standard method of BSI diagnosis, but it takes at least 24 hours to obtain a result, which seriously affects the efficacy of early diagnosis and clinical treatment (3). Although the newly developed molecular diagnosis method—polymerase chain reaction (PCR), is rather rapid, this technique is expensive, time-consuming, and requires special equipment, which limits its routine application in clinical laboratory (4).
Serum procalcitonin (PCT) is one of the alternative inflammation markers in the rapid BSI inspection. PCT is a procalcitonin substance without hormone activity which contains 116 amino acids. The secretion of PCT increases significantly after a stimulation of various of inflammations and infections (5). Recent studies showed that PCT was a promising inflammatory marker, which can distinguish systemic bacterial infection from other types of infections, judge the severity of bacterial infection, estimate the therapeutic effect and prognosis of BSI, and guide the use of antibiotics (6-9). Some recent studies have further found that PCT can distinguish different types of pathogenic bacteria on the basis of Gram staining in patients with BSI (1,10,11), providing rapid support for the preliminary selection of antibiotics. However, other studies did not show these findings (12,13). This indicates that distinguishing different types of pathogenic infections could not be determined by PCT test alone. Currently, the reason of the divergences about whether the PCT value could distinguish different pathogen types or not mainly lies on the differences in the research population, the criteria of inclusion, the acquisition time of PCT values and the interpretation of the results. However, there is no research focusing on the impact of antibiotics on the PCT value. In addition, Watanabe and his colleges (14) found that the PCT values of the BSI caused by multidrug-resistant organism (MDRO) were higher than that of the BSI caused by sensitive bacteria. However, the sample size in this study is too small, which requires further studies to confirm the conclusion.
The aims of this study are to examine (I) the association between PCT values of patients with BSIs and pathogen types; (II) the impact of MDRO infections on PCT values; and (III) the impact of antibiotics usage on the peak distribution on PCT values.
Methods
Study population
We retrospectively reviewed the medical files of all patients admitted to the First Affiliated Hospital of Nanjing Medical University (Nanjing, China) for subsequent BSI between January 1, 2013 and May 31, 2018. Data of 1,747 patients were collected. Inclusion criteria: diagnosed with BSI; having been given at least two times of PCT during hospitalization; showing only single pathogen after BC, or only one definite local focus of infection except for BSI (this focus was caused by the same pathogen to that cultured). Exclusion criteria: polymicrobial cultures separated; aged less than 18 years old; and BC contaminated.
Definitions
BSI is defined as the pathogen is discovered in one or more BCs from a patient with infection signs (such as fever, chills, and sweats) with or without local signs and symptoms. Patients with polymicrobial cultures were not eligible. Coagulase-negative staphylococci (CNS), Corynebacterium spp., and other skin commensals are considered as contaminants in isolating BCs. Diagnosis of CNS-related BSI was done based on the strains isolated from two BCs taken at two different time points and their having similar antibiogram (15).
Study design
The enrolled patients with BSI were divided into two groups: one group without definite focus of infection and one group with definite focus of infection. The patients without definite focus of infection were further divided into Gram positive group, Gram negative group and fungal group according to Gram staining results. The patients with definite focus of infection were further divided into lower respiratory tract infection group, infectious endocarditis group, urinary tract infection group, central nervous system infection group, skin-soft tissue infection group and upper respiratory tract infection group according to the location of focus.
Measurement of PCT level
Serum PCT levels were measured via an electrochemiluminescent immunoassay using Elecsys reagent, Elecsys BRAHMSPCT (Roche Diagnostics Shanghai Co., Ltd., Shanghai, China) and Cobas 8000 system (Roche Diagnostics, Rotkreuz, Switzerland), according to manufacturer’s recommendations. The lower limit of detection was 0.02 ng/mL and the functional assay sensitivity was 0.06 ng/mL.
Statistical analysis
Data analysis was conducted using SPSS 23 software (IBM, Armonk, USA). Discrete variables were described as percentage (%) and continuous variables as the mean with SD or medians with interquartile range (IQR) between 25th and 75th percentiles, as appropriate. The Chi-square test or Fisher’s exact test was used for the comparison between categorical variables. For the nonparametric data, comparisons between two datasets were made using the Man-Whitney U test and comparisons between three or more datasets were made using the Kruskal-Wallis H test. Receiver operation characteristics (ROC) curve analysis was used to define the diagnostic ability of various PCT cut-offs. Youden’s indices were calculated to find the best discriminatory cut-off. The threshold for significance was set at P<0.05.
Results
Epidemiological information of patients
During the study period, 1,747 patients with BSI were screened and 1,196 patients were excluded from the study. A total of 551 bacteremia patients meeting the inclusion criteria were enrolled in the study. Their basic clinical data were included in Table 1. In our cohorts, 314 bacteremia patients without definite focus of infection and 237 bacteremia patients with definite focus of infection were introduced into the final analysis. The average age of the patients was 59.3 years (IQR, 46.9–69.6 years); 59.5% were males; the community-acquired infection accounted for 63.2%; the average length of stay in hospital was 15 days; 72.6% of the patients were from department of medicine; BC indicated that 60.3% of them were Gram negative.
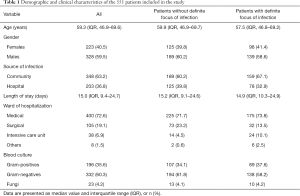
Full table
PCT values associated with Gram staining results of bacteremia patients without definite focus of infection
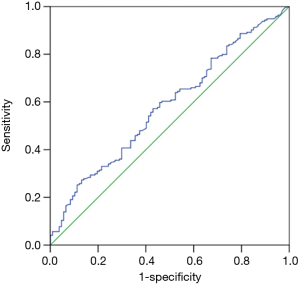
PCT median value according to Gram staining was shown in Figure 1. The number of bacteremia patients with Gram-positive bacteria, Gram-negative bacteria, and fungal infection was 107, 194 and 13, respectively. PCT median value of Gram-negative bacteria (1.16 ng/mL, IQR, 0.25–8.93 ng/mL,) was significantly higher than that observed in Gram-positive bacteria (0.51 ng/mL, IQR, 0.16–3.4 ng/mL, and P=0.02). However, there were no significant difference in PCT median value between Gram-negative bacteria or Gram-positive bacteria and fungal infection (0.41 ng/mL, IQR, 0.1–1.67 ng/mL, and P=0.135, 0.654, respectively). The ROC curve of PCT values for Gram-positive and Gram-negative bacteria infections showed that the area under AUC curve was 0.581 (95% CI, 0.51–0.65, P=0.02). When the cut-off value was 7.54 ng/mL, we found a sensitivity of 86.9%, a specificity of 27.3%, a PPV of 39.7%, an NPV of 79.7%, and a diagnostic accuracy of 48.5% (see Figure 2).
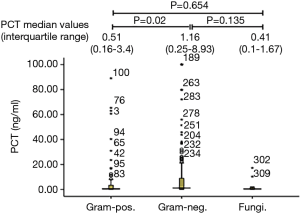
PCT values associated with the pathogen type in bacteremia patients without definite focus of infection
Median (quartile) and average PCT values caused by different microbial species of Gram-positive bacteria, Gram-negative bacteria and fungi were shown in Table 2. No significant difference was found between different types of Gram-positive bacteria, and so did Gram-negative bacteria and fungi (all P>0.05). For Gram-positive bacteria, PCT values did not differ among Enterococcus, Streptococcus and Staphylococcus (data not shown). For Gram-negative bacteria, PCT median values between Enterobacteriaceae [1.51 (0.28–10.48)] and non-fermentative bacteria [0.99 (0.24–4.14)] were also not statistically significant (P=0.340).
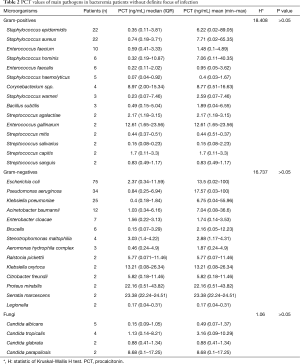
Full table
PCT values associated with focal location in bacteremia patients with definite focus of infection
PCT median (four quantile) values of bacteremia patients with seven different focus of infection were shown in Figure 3 and Table 3. Among seven different foci of infection, PCT value of patients with abdominal infection was significantly higher than that of patients with infective endocarditis (P=0.01). No significant difference was found between the other foci of infection.
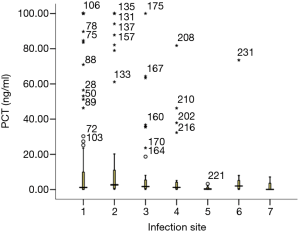

Full table
Further, PCT values of Gram-positive bacteria and Gram-negative bacteria in every focus were compared (Table 4). PCT values of Gram-negative bacteria were significantly higher than those of Gram-positive bacteria in patients with lower respiratory tract infection, abdominal, urinary tract infection, but not in those with lower respiratory tract infection (P=0.003, 0.039, 0.025 and 0.664, respectively). The number of Gram-positive or Gram-negative bacteria in patients with infective endocarditis, skin and soft tissue infections, and central nervous system infections were very low (data not shown).
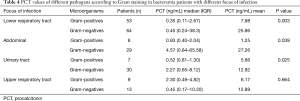
Full table
ROC curve analysis of PCT values of Gram-negative and Gram-positive bacteria in bacteremia patients with definite focus of infection
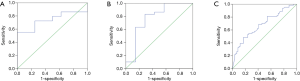
The ROC curve of PCT for distinguishing Gram-positive from Gram-negative bacteria in abdominal infection showed that the area under AUC curve was 0.741 (95% CI, 0.58–0.90, P=0.039). A cut-off value of 3.01 ng/mL for PCT achieved a sensitivity of 100%, a specificity of 55.2%, a PPV of 100%, an NPV of 38% and a diagnostic accuracy of 64.9% (Figure 4A). The ROC curve of PCT for distinguishing Gram-positive from Gram-negative bacteria in urinary tract infection showed that the area under AUC curve was 0.776 (95% CI, 0.54–1.01, P=0.025). A cut-off value of 0.574 ng/mL for PCT achieved a sensitivity of 83%, a specificity of 71.4%, a PPV of 50%, an NPV of 92.6% and a diagnostic accuracy of 81.1% (Figure 4B). The ROC curve of PCT for distinguishing Gram-positive from Gram-negative bacteria in upper respiratory tract infection showed that the area under AUC curve was 0.682 (95% CI, 0.58–0.78, P<0.001). A cut-off value of 5.99 ng/mL for PCT achieved a sensitivity of 83.1%, a specificity of 48.4%, a PPV of 57.1%, an NPV of 48.4% and a diagnostic accuracy of 64.1% (Figure 4C).
Comparison of PCT values between MDROs and sensitive bacteria in bacteremia patients
Two strains of carbapenem-resistant Klebsiella pneumoniae (CR-KP), two strains of carbapenem-resistant Escherichia coli (CR-E. coil), six strains of carbapenem-resistant Acinetobacter baumannii (CR-AB), two strains of carbapenem-resistant Pseudomonas aeruginosa (CR-PA), seven strains of methicillin-resistant Staphylococcus aureus (MRSA), and two strains of vancomycin-resistant Enterococcus (VRE) were detected in bacteremia patients without definite focus of infection. Comparative results of PCT values between MDROs and their corresponding sensitive bacteria were shown in Table 5. Differences in PCT values between MDROs and sensitive bacteria were not statistically significant (P>0.05).
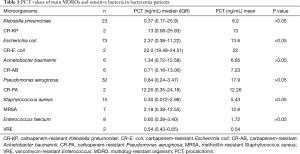
Full table
The number of MDROs strains in bacteremia patients with definite focus of infection including abdominal, urinary tract, upper respiratory tract, infective endocarditis, skin and soft tissue and central nervous system were small (data not shown). Four strains of CR-AB, nine strains of MRSA and two strains of VRE were detected in foci of lower respiratory tract infections. The PCT values of MDROs and sensitive bacteria were shown in Table 6. Difference in PCT value between them were also not statistically significant (P>0.05).

Full table
Analysis of PCT peak value distribution
The time point when the BC was collected was set as baseline point. If the PCT peak appears after the baseline point, then the difference value was positive; if PCT peak appeared before the base point, the difference value was negative. The result showed average difference between the time point of PCT peak and the baseline point was −0.74 days. From the time concentration trend, 70% of the time points of PCT peak value was distributed in 48 hours before and after BC collection. The distribution of time points of PCT peak value and time points of collecting BC were shown in Figure 5. As shown, 44.3% of PCT peak values appeared before BC collection, 33.8% after BC collection. The distribution of peak values for specific pathogen according to Gram staining was shown in Table 7.
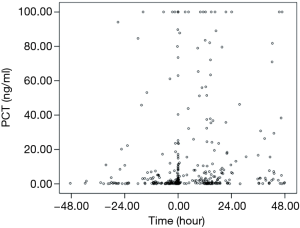
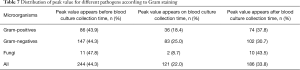
Full table
Of 551 patients, 348 (63.2%) did not use antibiotics and 203 (36.8%) did. In patients having not used antibiotics, 51.7% of PCT peak values appeared before BC collection and 22.7% after BC sampling. However, in patients with antibiotics use, 31.5% appeared before BC sampling and 52.7% after BC collection (Table 8). The proportion of PCT peak values in patients with antibiotics use before BC collection was significantly lower than that in patients without antibiotics use (31.5% vs. 51.7%, P<0.001), while the proportion of PCT peak value in patients with antibiotics use after BC collection was significantly higher than that in patients without antibiotics use (52.7% vs. 22.7%, P<0.001). For the patients who used antibiotics before BC collection, the antibiotics were used in a longer term (from 1 to 3 days or more), and the proportion of PCT peak distribution before BC collection had a falling trend, but the proportion of PCT peak distribution after BC collection had an increasing trend. Meanwhile, the higher proportion of antibiotics combined before BC collection (from single use to combination of five kinds), the lower proportion of PCT peak distribution before BC collection, and the higher proportion of PCT peak distribution after BC collection.
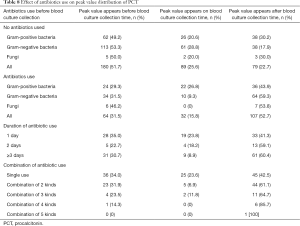
Full table
Discussion
The BSI, with an incidence on the rise and reported to be 0.6–0.8% (16), threatens the lives of patients in clinical work. BC can be used to effectively and accurately diagnose BSI, but it has some limitations, such as long-term incubation, being easily contaminated, multiple times of blood sampling and low positive rate of pathogens (17). At thxe same time, the frequent misdiagnose can fail the differential diagnosis and delay the treatment. With the development of diagnostic techniques, PCT, as a new inflammatory marker, has been widely used to distinguish bacterial from non-bacterial infections (18).
Current studies have confirmed that PCT is a sensitive inflammatory marker. Even under a low inflammatory state, PCT recognition of infectious and non-infectious inflammatory response is still more efficient than other inflammatory markers, especially for patients with severe microbial infection and BSI (19,20). A person without infection has a low serum PCT level, when BSI occurs, inflammatory factors and bacterial endotoxins stimulate the secretion of PCT through different Toll-like receptor signaling pathways (21). Animal experiments showed that PCT could be detected in the liver, kidney, spleen and lung exposed to endotoxin, bacterial lipopolysaccharide and Escherichia coli for several hours (22). This fact is also supported by a clinical research in which PCT value increased in the tissues injected with endotoxin (23). It was reported that the persistent high level of PCT in the blood was significantly influenced by endotoxin and lipopolysaccharide (24,25). Studies also found that BSI caused by Gram-negative bacteria led to a significantly higher PCT level than that by Gram-positive bacteria (26). The mechanism is that Gram-negative bacteria and Gram-positive bacteria recognize Toll-like receptor 4 and Toll-like receptor 2 on the cell surface by lipopolysaccharide and lipoteichoic acid, respectively, and subsequently induce the release of different cytokines and the different PCT levels (27,28).
Previous studies on the identification of pathogens of BSI by PCT have got inconsistent results. For example, Brodská et al. (1) revealed that infections caused by Gram-positive bacteria, Gram-negative bacteria and fungi could be distinguished by PCT values, and the PCT value of fungal infection was significantly lower than those of other pathogens. This study selected ICU patients as the main study population and the bacteremia patients with positive staining results as the experimental group. Other infections except for BSI were not considered as influential factors, but multi-site infections caused by a variety of pathogens might affect the PCT values. Mencacci et al. (4) have proved that PCT values can replace BC and PCR technology in the diagnosis of candidemia. This study focused on patients with positive BC and body temperature close to or higher than 37 °C. The foci of infection in most patients were unknown, and may be combined with multi-site infections. If isolated pathogens were not consistent with the BC pathogens, then the PCT value could not accurately reflect the pathogen from BSI, so the ability of PCT to distinguish BSI pathogen was still insufficient. In most studies, collection time points of PCT were diverse, sampled either simultaneously as BC or on the same day or within 48 hours. The collection of BC is determined by healthcare workers and mainly based on clinical manifestations and laboratory indicators. It may be subjective and may not be consistent with the growth and reproduction time of pathogens. So the measured PCT values might not fully reflect the actual PCT values indicating the levels the pathogen (11). In addition, the early use of antibiotics may affect the actual PCT value. Our study limited the inclusion criteria to a sole BSI or a local infection secondary to BSI (the local infection pathogen was the same as the BC pathogen), and took the peak PCT value as the actual value. Our study confirmed that the PCT median value of bacteremia patients with Gram-negative bacteria infection was significantly higher than that of Gram-positive bacteria infection. These findings are consistent with the previously published literature (1,10,11). But because the specificity was only 27%, the 0.581 of area under the curve was unsatisfactory and could not guide clinical diagnosis and treatment, which was in consistent with conclusions from Thomas-Rüddel et al. (13).
In 2014, the World Health Organization (WHO) announced that antimicrobial resistance is a global problem that needs to be addressed urgently (29). MDROs have high morbidity and mortality, and treating them is difficult and costly, which seriously threatens global security. The irrational use of antibiotics results in a global increase in the incidence and prevalence of MDROs (30). Japanese expert Watanabe et al. (14) found that the PCT value of patients infected with ESBL positive strains was significantly higher than that with ESBL negative strains, which indicated that the PCT values can distinguish ESBL positive strains from negative strains, but the population enrolled in their study was insufficient. However, the present study found different results. For the patient either without or with definite focus of infection, the PCT values of carbapenem-resistant Enterobacteriaceae (CRE), CR-AB, CR-PA, MRSA and VRE infection had no significant differences from those of sensitive bacterial infection. These results suggest that PCT levels cannot yet distinguish MDROs from corresponding sensitive bacteria in bacteremia patients. Therefore, we speculate that there may be difference in the intensity of inflammation stimulation between MDROs and corresponding sensitive bacteria.
Previous studies have confirmed that PCT values can be used in the diagnosis of local infections such as skin and soft tissue abscess, diabetic foot infection, infectious arthritis and osteomyelitis (31). Yan et al. (11) showed that, for secondary bacteremia patients, the PCT value of abdominal infection was higher than that of endocarditis and pneumonia, and PCT value of urinary tract infection was higher than that of pneumonia, catheter-related infection and endocarditis. Yu et al. (32) discovered that there were differences in PCT values among different sites of infection. PCT values of abdominal infection (8.32 ng/mL) and biliary tract infection (5.98 ng/mL) were higher; those of chest infection (1.39 ng/mL) and brain infection (0.46 ng/mL) were lower. Our results showed that PCT values of abdominal, skin and soft tissue, and lower respiratory tract infection sites were relatively high, but the differences were not statistically significant. These data suggest that, for patients with local infection complicated by BSI, it is unable to identify the focus of infection by serum PCT values. According to Gram staining, a subgroup analysis on the infection sites (lower respiratory tract, abdomen, urinary tract and upper respiratory tract) indicated PCT values could distinguish Gram-positive bacteria from Gram-negative bacteria, except for upper respiratory tract infection. Besides lower respiratory tract infection, the difference between Gram-positive bacteria and Gram-negative bacteria in abdominal and urinary tract infections can be well distinguished. In patients with abdominal infection was, a PCT value of 0.74 ng/mL achieved a sensitivity of 100%, and in patients with urinary tract infection, a PCT value of 0.78 ng/mL achieved a sensitivity of 83.3%. The above results confirmed that although we can distinguish Gram-positive bacteria and gram-negative bacteria in some specific sites of infection, but different cut-off values of PCT should be adopted for different sites of infections. It is also suggested that PCT can be used to differentiate pathogens in patients with local infection combined with BSI, and the efficiency of differentiation is better than that in patients with BSI alone.
PCT is a precursor of calcitonin glycoprotein containing 116 amino acids. Its half-life is relatively short (25–30 hours). Its value increases in 2–3 hours after bacterial infection and shows a continuous dynamic change trend in serum (14). Relevant studies have reported that the continuous increase or decrease of PCT was closely related to the severity of bacterial infection and the inflammatory response (33,34). PCT levels in patients are dynamic, and the time points of PCT collection and BC are greatly affected by the subjective consciousness of healthcare workers. Many peak value time points of PCT do not coincide with BC collection time points. In previous studies, the tested PCT value did not absolutely reflect the real PCT value induced by the special pathogen based on the research design in which BC and PCT value were performed at the same time, so the cut-off values of ROC curve from different studies varied greatly. The reason may be that the PCT value was not consistent with the actual value, and its collection time was not reliable (35). This study confirmed that 78.1% of peak values of PCT did not appear at the time point of BC collection, indicating that when BC and PCT value were collected at the same time, we may not obtain the actual PCT value induced by the special pathogen, so this value cannot represent the level of the pathogen.
In addition to the analysis of the peak time of PCT, we analyzed whether antibiotics were used before BC collection, and the results showed that antibiotics caused more PCT peaks appearing after BC collection time. At the same time, the longer use of antibiotics before BC collection time, the higher proportion of antibiotics combination, and the greater probability of PCT peak value occurring after BC collection. The above data fully showed that the antibiotics use can affect the distribution of PCT values. If this effect is not considered, the PCT value of the pathogen is not original. The reason is not very clear, but one may be that antibiotics use can lead to the death of pathogens, and dead bacteria release a large amount of endotoxins, thus inducing a high level of PCT secretion (21).
This study has the following advantages. Firstly, we strictly control the inclusion criteria. The bacteremia patient without definite focus of infection, any other site infection, is excluded, which ensures that the PCT value and BSI pathogens are clearly related. For patients with definite focus of infection, there is only one infection site besides BSI, and the local infection and BSI are caused by the same pathogen. This method ensures the correlation not only between PCT value and pathogen, but also between PCT value and focus of infection. Secondly, according to the distribution of PCT peak value, we study the relationship between PCT value and pathogen infection which is closer to the real world. Thirdly, this study analyzes the effect of antibiotics use on PCT value for the first time, which provides a new idea to study the correlation between PCT value and pathogen infection in the future. However, there are some limitations in this study: first, this study is a retrospective research and its efficiency is relatively low. Second, the population studied is screened under strict standards, and the research conclusions may not provide practical guidance for complicated clinical cases. Third, the basic condition of study population is not analyzed. It is difficult to exclude other causes of abnormal elevation of PCT except for pathogens. Fourth, although the study shows PCT value cannot distinguish bacteremia patients with MDROs from susceptible bacteria, but due to the number of MDROs cases is relatively small, this conclusion needs further confirmation of larger sample studies in the future.
Conclusions
PCT value is not only determined by Gram-positive bacteria or Gram-negative bacteria, but also specific pathogens, infection sites. Further, basic diseases, treatment measures such as antibiotics use should also be considered as factors.
Acknowledgements
Funding: This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (JX10231803).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (approval number: 2019-SR-098). Since the study was a retrospective analysis, the ethics committee waived the need for consent.
References
- Brodská H, Malíčková K, Adámková V, et al. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med 2013;13:165-70. [Crossref] [PubMed]
- Prowle JR, Echeverri JE, Ligabo EV, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Critical Care 2011;15:R100. [Crossref] [PubMed]
- Seki M, Takahashi H, Yamamoto N, et al. Polymerase chain reaction based active surveillance of MRSA in emergency department patients. Infect Drug Resist 2015;8:113-8. [Crossref] [PubMed]
- Mencacci A, Leli C, Cardaccia A, et al. Procalcitonin predicts real-time PCR results in blood samples from patients with suspected sepsis. PLoS One 2012;7:e53279. [Crossref] [PubMed]
- Christ-Crain M, Müller B. Procalcitonin in bacterial infections--hype, hope, more or less? Swiss Med Wkly 2005;135:451-60. [PubMed]
- Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:426-35. [Crossref] [PubMed]
- Giannakopoulos K, Hoffmann U, Ansari U, et al. The use of biomarkers in Sepsis: a systematic review. Curr pharm Biotechnol 2017;18:499. [Crossref] [PubMed]
- Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care 2017;7:114. [Crossref] [PubMed]
- Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017;10:CD007498. [PubMed]
- Pieralli F, Corbo L, Torrigiani A, et al. Usefulness of procalcitonin in differentiating Candida and bacterial blood stream infections in critically ill septic patients outside the intensive care unit. Intern Emerg Med 2017;12:629-35. [Crossref] [PubMed]
- Yan ST, Sun LC, Jia HB, et al. Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria. Am J Emerg Med 2017;35:579-83. [Crossref] [PubMed]
- Hoenigl M, Raggam RB, Wagner J, et al. Procalcitonin fails to predict bacteremia in SIRS patients: a cohort study. Int J Clin Pract 2014;68:1278-81. [Crossref] [PubMed]
- Thomas-Rüddel DO, Poidinger B, Kott M, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care 2018;22:128. [Crossref] [PubMed]
- Watanabe Y, Oikawa N, Hariu M, et al. Ability of procalcitonin to diagnose bacterial infection and bacteria types compared with blood culture findings. Int J Gen Med 2016;9:325-31. [Crossref] [PubMed]
- Clinical and Laboratory Standards Institute. Principles and procedures for Blood Cultures. Approved Guideline. M47A. Wayne, PA, 2007.
- Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, et al. Sepsis: A Review of Advances in Management. Adv Ther 2017;34:2393-411. [Crossref] [PubMed]
- Siegel JD, Rhinehart E, Jackson M, et al. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control 2007;35:S165-93. [Crossref] [PubMed]
- Hoeboer SH, Pj VDG, Nieboer D, et al. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015;21:474-81. [Crossref] [PubMed]
- Falcão Gonçalves P, Menezes FL, Duque PI. Procalcitonin as Biomarker of Infection: Implications for Evaluation and Treatment. Am J Ther 2017;24:e243-9. [Crossref] [PubMed]
- Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206-17. [Crossref] [PubMed]
- Kumar S, Ingle H, Prasad DV, et al. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol 2013;39:229-46. [Crossref] [PubMed]
- Matwiyoff GN, Prahl JD, Miller RJ, et al. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res 2012;61:401-9. [Crossref] [PubMed]
- Morgenthaler NG, Struck J, Chancerelle Y, et al. Production of procalcitonin (PCT) in non-thyroidal tissue after LpS injection. Horm Metab Res 2003;35:290-5. [Crossref] [PubMed]
- Castelli GP, Pognani C, Cita M, et al. procalcitonin as a prognostic and diagnostic tool for septic complications after major trauma. Critical Care Medicine 2009;37:1845-9. [Crossref] [PubMed]
- Ryu YH, Baik JE, Yang JS, et al. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int Immunopharmacol 2009;9:127-33. [Crossref] [PubMed]
- Cortegiani A, Russotto V, Montalto F, et al. Procalcitonin as a marker of Candida species inspection by blood culture and polymerase chain reaction in septic patients. BMC Anesthesiology 2014;14:9. [Crossref] [PubMed]
- Gao H, Evans TW, Finney SJ. Bench-to-bedside review: sepsis, severe sepsis and septic shock-does the nature of the infecting organism matter? Crit Care 2008;12:213. [Crossref] [PubMed]
- Leaver S, Burke Gaffney A, Evans TW. Gram-positive and Gram-negative sepsis: two disease entities? In: Vincent JL. editor. Intensive Care Medicine: Annual Update 2008. New York: Springer, 2008:395-403.
- Hawkey PM. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect 2015;89:241-7. [Crossref] [PubMed]
- Nordmann P, Naas T, Poirel L.. Global Spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011;17:1791-8. [Crossref] [PubMed]
- Saeed K, Ahmad N, Dryden M. The value of procalcitonin measurement in localized skin and skin structure infection, diabetic foot infections, septic arthritis and osteomyelitis. Expert Rev Mol Diagn 2014;14:47-54. [Crossref] [PubMed]
- Yu Y, Li XX, Jiang LX, et al. Procalcitonin levels in patients with positive blood culture, positive body fluid culture, sepsis, and severe sepsis: a cross-sectional study. Infect Dis (Lond) 2016;48:63-9. [Crossref] [PubMed]
- Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012;55:651-62. [Crossref] [PubMed]
- Guo SY, Zhou Y, Hu QF, et al. Procalcitonin Is a Marker of Gram-Negative Bacteremia in patients With Sepsis. Am J Med Sci 2015;349:499-504. [Crossref] [PubMed]
- Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evid Based Child Health 2013;8:1297-371. [Crossref] [PubMed]


