
Metachronous renal cell carcinoma: an unbeatable leviathan?
Renal cell carcinoma (RCC) is a heterogeneous tumor, even within the same histological type (1). This feature, combined with non-modifiable (age, sex, race, genetic), and modifiable (family, and smoking history, hypertension, drugs) predisposing factors, increases the risk to develop metachronous RCC at long-term (2). Besides, concerns still remain about what a metachronous tumor is, and on what features allow to recognize it as metachronous rather than recurrence (3,4). Understanding the predisposing factors, and find a univocal portrayal of “metachronous” might shift the history of this condition. Nevertheless, literature have not found an answer to these questions yet, and evidence remains sparse. Because of this, we strongly applaud the authors for their effort to provide a further insight on this controversial topic fueling the debate, and giving hint for future studies.
Syed et al. assessed the risk factors for metachronous bilateral RCC within the Surveillance, Epidemiology, and End Result (SEER) database, carrying out a rigorous analysis on 80,403 cases of RCC. Among these, 1,063 patients presented metachronous renal tumor. The authors demonstrated a cumulative incidence at 10, 20 and 30 years of 1.5%, 3.1%, and 4.7%, respectively. Younger age, male gender, black race, and papillary histology demonstrated to predispose to contralateral metachronous RCC. Notably, this risk remained permanent even at a follow-up time ≥10 years (5). Interestingly, the authors included only those patients diagnosed for secondary renal tumor at 12 months from the first one, this as means to exclude those cases which could be considered recurrence. Because of the SEER dataset setting, the authors were not allowed to account for anamnestic data which might permit to evaluate further confounders. One of these is the multifocality which seems to be expression of primary tumor metastasis rather than a new metachronous one. Assessing renal masses biopsy of 20 patients, Kume demonstrated that 5 presented subclinical Von-Hippel Lindau (VHL) mutation, and 3 the same mutation on both sides, confirming the same origin (6). In spite of the aforementioned evidences, Syed’s findings were consistent with those of a large multi-institutional study which denoted that multifocality of the first, and of the second RCC, VHL disease, and a family history positive for RCC were more likely to appear in patients with metachronous kidney carcinoma (2). The above-mentioned results let us to suppose that general population might present the same characteristics. Consequently, those patients with predisposing factors should be addressed to strict follow-up, this considering the correlation of metachronous tumor with younger age also. A recent study on genomic of RCC demonstrated that age was related to downregulation of extracellular matrix organization gene, which is involved angiogenesis, tumor growth, and metastatic spread. This might explain the higher odds of metachronous tumor in younger patients (7). One newsworthy result of the current analysis is the correlation of papillary histology with the risk of metachronous kidney tumor. This finding can be due to the association of papillary variance with genetic syndromes which predispose to tumor development. Indeed, papillary RCC seems to be more frequent in patients affected by PTEN Hamartoma Tumor Syndrome (8), and VHL syndrome (9), but we can only surmise this because of the absence of this data in the SEER dataset.
Regarding the time to late tumor development, the authors underlined that the risk seems not to decrease with the time flow reporting the same value at ≥10 years distance from the first diagnosis. The long-term risk was already shown in previous population-based studies. Within the SEER registry, Rabbani et al. assessed a sample of 357 metachronous tumors out of 43,483. The authors found a higher risk for black patients, especially in the first 5 years, but with a higher risk beyond ten years as well (10). On the contrary, Wiklund et al. showed a decreased risk trend in the years from the assessment of the Norway and Sweden dataset, but on a small cohort of patients, and on a shorter average follow-up time (11) (Table 1). Given the risk of late metachronous RCC development, the Syed’s report generates a question about whether it is time to shift the management of RCC.
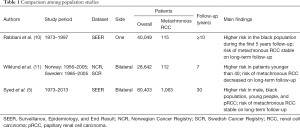
Full table
In the era of nephron sparing surgery (NSS) (12,13), radical nephrectomy (RN) still represents a widely used treatment option for RCC, even for low stage disease (14). This trend exposes patients to the risk of a more complex procedure on solitary kidneys in the future, with the functional, and oncological well-known sequelae (15). Thus, the risk of late metachronous RCC should induce to stratify patients according to their probability of secondary renal neoplasm, pushing the indication to PN also to larger renal masses (16). Equally, if on one side PN offers kidney preservation, a repeated surgical procedure for secondary new onset RCC might translate into a more challenging tumor resection with consequent higher intraoperative, and post-operative complications. Watson et al. assessed repeated PN on a cohort of 124 patients showing that the procedure is feasible in well selected patients, which, however, have more odds of complications, especially urine leakage (17).
All these considerations indicate the necessity to change patients counseling, and selection. Indeed, presence of predisposing factors to metachronous RCC should lean forward to conservative management opting for active surveillance (AS), or less invasive procedures like ablation therapy (18,19). Finally, the results of this study claim the need to predetermine the genetic, molecular, and histological nature of RCC to establish the most effective treatment algorithm. The “omics” seem to be promising tools which might help to establish a management perfectly tailored on patients’ risks (20).
In summary, RCC can be an aggressive disease even after several years from its initial treatment. Patients presenting high risk for metachronous RCC need to be better characterized, and life-long follow-up could be justified in this subset. While we commend the authors for their work again, further studies are needed to solve unsettled questions on RCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Inamura K. Renal cell tumors: understanding their molecular pathological epidemiology and the 2016 who classification. Int J Mol Sci 2017;18:E2195. [Crossref] [PubMed]
- Klatte T, Patard JJ, Wunderlich H, et al. Metachronous bilateral renal cell carcinoma: risk assessment, prognosis and relevance of the primary-free interval. J Urol 2007;177:2081-6. [Crossref] [PubMed]
- Antonelli A, Furlan M, Sodano M, et al. Features, risk factors and clinical outcome of "very late" recurrences after surgery for localized renal carcinoma: A retrospective evaluation of a cohort with a minimum of 10 years of follow up. Int J Urol 2016;23:36-40. [Crossref] [PubMed]
- Antonelli A, Furlan M, Tardanico R, et al. Features of ipsilateral renal recurrences after partial nephrectomy: a proposal of a pathogenetic classification. Clin Genitourin Cancer 2017;15:540-7. [Crossref] [PubMed]
- Syed JS, Nguyen KA, Holford TR, et al. Risk factors for metachronous bilateral renal cell carcinoma: A surveillance, epidemiology, and end results analysis. Cancer 2019;125:232-8. [Crossref] [PubMed]
- Kume H, Oda H, Nakatsuru Y, et al. Genetic identification of bilateral primary or metastatic nonpapillary renal cell carcinoma. BJU Int 2000;86:208-12. [Crossref] [PubMed]
- Feulner L, Najafabadi HS, Tanguay S, et al. Age-related variations in gene expression patterns of renal cell carcinoma. Urol Oncol 2019;37:166-75. [Crossref] [PubMed]
- Mester JL, Zhou M, Prescott N, et al. Papillary renal cell carcinoma is associated with PTEN hamartoma tumor syndrome. Urology 2012;79:1187.e1-7. [Crossref] [PubMed]
- Williamson SR, Cheng L. Do clear cell papillary renal cell carcinomas occur in patients with von Hippel-Lindau disease? Hum Pathol 2015;46:340-1. [Crossref] [PubMed]
- Rabbani F, Herr HW, Almahmeed T, et al. Temporal change in risk of metachronous contralateral renal cell carcinoma: influence of tumor characteristics and demographic factors. J Clin Oncol 2002;20:2370-5. [Crossref] [PubMed]
- Wiklund F, Tretli S, Choueiri TK, et al. Risk of bilateral renal cell cancer. J Clin Oncol 2009;27:3737-41. [Crossref] [PubMed]
- Mir MC, Derweesh I, Porpiglia F, et al. Partial nephrectomy versus radical nephrectomy for clinical t1b and t2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol 2017;71:606-17. [Crossref] [PubMed]
- Antonelli A, Minervini A, Sandri M, et al. Below safety limits, every unit of glomerular filtration rate counts: assessing the relationship between renal function and cancer-specific mortality in renal cell carcinoma. Eur Urol 2018;74:661-7. [Crossref] [PubMed]
- Xia L, Talwar R, Taylor BL, et al. National trends and disparities of minimally invasive surgery for localized renal cancer, 2010 to 2015. Urol Oncol 2019;37:182.e17-7. [Crossref] [PubMed]
- Minervini A, Mari A, Borghesi M, et al. The occurrence of intraoperative complications during partial nephrectomy and their impact on postoperative outcome: results from the RECORd1 project. Minerva Urol Nefrol 2019;71:47-54. [Crossref] [PubMed]
- Bertolo R, Autorino R, Simone G, et al. Outcomes of robot-assisted partial nephrectomy for clinical t2 renal tumors: a multicenter analysis (ROSULA Collaborative Group). Eur Urol 2018;74:226-32. [Crossref] [PubMed]
- Watson MJ, Sidana A, Diaz AW, et al. Repeat robotic partial nephrectomy: characteristics, complications, and renal functional outcomes. J Endourol 2016;30:1219-26. [Crossref] [PubMed]
- Gupta M, Patel HD, Pierorazio PM. Active surveillance of small renal masses: a safe management strategy for select patients. Eur Urol 2018;74:165-6. [Crossref] [PubMed]
- Uhlig J, Kokabi N, Xing M, et al. Ablation versus resection for stage 1a renal cell carcinoma: national variation in clinical management and selected outcomes. Radiology 2018;288:889-97. [Crossref] [PubMed]
- Anele UA, Hampton LJ, Grob MB, et al. Prediction of aggressive histology: the ongoing dilemma of renal masses in the "omics" era. Eur Urol 2018;74:498-500. [Crossref] [PubMed]


