
Placement of markers to assist minimally invasive resection of peripheral lung lesions
Introduction
Pulmonary nodules are well-circumscribed lesions that are surrounded by pulmonary parenchyma and measure <3 cm. These nodules can be solitary or multiple, solid or subsolid (1,2), and more importantly, have the potential of being malignant. The incidence of pulmonary nodules in the United States has been reported to be 1.5 million and is expected to rise with current screening guidelines and advances in imaging technology (3).
Peripheral lung lesions (PLLs) are lung nodules located in the periphery of the lung and are typically difficult to biopsy. Their incidence in the general population has not been described, but they also have malignant potential.
Current guidelines for solid nodules recommend tissue sampling for single indeterminate lesions that are ≥8 mm in some low and high-risk patients, and in those patients in which there is evidence of nodule growth during the recommended follow up period (4). The American College of Chest Physicians (ACCP) recommend surgical lung biopsy for indeterminate nodules that are >8 mm in diameter, have a high probability of malignancy, are hypermetabolic or positive by functional imaging tests, when a non-surgical biopsy is suspicious for malignancy, or when a fully informed patient prefers a definitive diagnostic procedure (5). Surgical biopsy is also recommended as an option for part solid (>50% ground glass) nodules that are >0.8 cm in diameter that persist 3 months after an initial CT scan and those part solid nodules that are >1.5 cm in diameter (5).
Tissue sampling techniques include CT guided needle aspiration, bronchoscopy with transbronchial biopsy, either by conventional fluoroscopy or guided with electromagnetic navigation bronchoscopy (ENB), radial endobronchial ultrasound (EBUS), or a combination of these. Surgical lung biopsy is the gold standard for the diagnosis and treatment of PPLs.
Minimally invasive thoracic surgery (MITS) is the preferred approach for surgical resection of PLLs. MITS includes video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS). Compared to open thoracotomy for surgical resection of PLLs, MITS has lower complication and mortality rates and is associated with less pain and a decrease in length of stay (6-8). RATS may have better outcomes than VATS but at a higher cost and operative time. This debate is an active area of investigation, with some evidence suggesting that there is no difference between VATS and RATS lobectomy and wedge resection (8-11).
Lung cancer continues to be the leading cause of cancer deaths, and the second most diagnosed cancer in the US. The use of low-dose chest CT scans for lung cancer screening has led to the detection of more PLL (12). Many of these lesions will undergo surgical resection for diagnosis and cure of early-stage lung cancers. Small PLL and those that are ground glass or semisolid or lesions that are not close to the visceral pleura may represent a challenge during VATS or RATS. Ground glass lesions or those not near the visceral pleura maybe difficult to locate (12). Various modalities have been developed to facilitate PLL identification during MITS by marking PLL.
We searched the Cochrane library and PubMed from 1990 to 2019 to obtain a comprehensive review of the different methods of placement of markers and tattoos for PLL. We used the following search criteria: Lung nodule, peripheral lung lesion, preoperative marking, thoracoscopic surgery, fiducial, dye, transthoracic, bronchoscopy, Virtual bronchoscopy, electromagnetic bronchoscopy.
Percutaneous placement of markers
Percutaneous techniques for the placement of lung markers for PLLs have proven to be effective and relatively safe (13). They are mostly performed under CT guidance. Local anesthesia and moderate sedation are frequently necessary for patient comfort and accuracy of marker placement.
Table 1 describes the profile for the most common percutaneous methods while Table 2 summarizes some of the landmark studies for each.
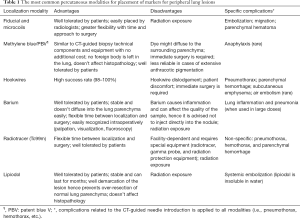
Full table

Full table
Percutaneous fiducials and microcoils placement
Fiducial markers are small metal spheres (typically gold), that can be placed in or near PLLs and can be localized intraoperatively under fluoroscopy. The technique of fiducial marker placement is similar to that of image-guided percutaneous biopsy (14) with a success rate of 95–98% (14,15). Microcoils for pleural marking consist of placing a coil with the distal end adjacent to the nodule and the proximal end coiled in the pleural space. This technique assumes that the surgeon will resect the tumor and coil together.
General complications of the percutaneous marker placement techniques include pneumothorax and hemothorax. Specific complications of fiducial markers are low, and the procedure is generally well tolerated (15). Fiducial embolization, migration, and parenchymal hematoma have been reported (14,15,28). One strategy to lower the rate of pleural migration is to deploy the marker into the parenchyma through longer path and not through the closest path to the pleura (15).
Microcoils are usually platinum-based and can be placed in or near the lesion of interest and then identified by fluoroscopy. Like fiducials, they differ from hookwires, in that there is no thread left protruding outside the skin, making fluoroscopy necessary for their identification during MITS. Finley et al., prospectively randomized 60 patients with lung nodules (≤15 mm) to either microcoil localization (29 patients) or no localization (27 patients). Four patients did not undergo surgery. The coil group had a higher rate of successful diagnosis with VATS than wedge resection alone (27/29 vs. 13/27; P<0.001), decreased operative time to nodule excision (37±39 vs. 100±67 minutes; P<0.001), and reduced stapler firings (3.7±2.0 vs. 5.9±31; P=0.003) with no difference in total cost [$4,903±2,518 (median, $3,930; range, $2,366–11,952) vs. $5,803±2,615 (median, $5,363; range, $1,975–11,282)]. Four patients in the microcoil group had a pneumothorax as a complication, but none of them required chest tube placement (16).
Percutaneous placement of methylene blue (MB)
PLL localization by MB is a safe and inexpensive procedure compared to other modalities. The technical aspects and the equipment needed for the procedure are similar to those of CT-guided needle aspiration (17,28). The closest distance between the chest wall and the nodule is identified and the dye is injected into or around the margins of the nodule and along the needle tract (lung, pleura and chest wall) during the withdrawal of the needle (18). Patients tolerate the procedure well as there is no foreign body left inside the lung parenchyma (17). Thoracoscopic identification rate of the nodule is 90–100% (17,18).
An important disadvantage of MB marking is diffusion of the dye into the surrounding lung parenchyma preventing precise identification of PLLs (17,18,22). Thoracoscopy should therefore begin soon after the localization procedure, ideally within 120–150 minutes (18). To prevent rapid diffusion, modified approaches have been described such as MB-stained autologous blood (29) and MB mixed with contrast and collagen (30).
An additional limitation of MB marking may occur when anthracotic pigmentation of the lung parenchyma is present. In these cases the dye may not be distinguishable from the surrounding lung during thoracoscopy (17). Anaphylaxis induced by MB has been reported but is extremely rare (31).
Percutaneous placement of hookwire
Hookwire placement is the oldest and the most common method used for PLL localization (22). The wire is introduced and deployed percutaneously and a thread is left outside the chest wall. The surgeon follows this tract in order to reach the lesion. Given the risk of pneumothorax, wire dislodgment, as well as patient discomfort and pain, the wire placement is usually performed right before the planned surgery. Some authors reported successful experience with leaving the wire in place for longer periods (20). The presence of interstitial lung disease is associated with increased risk of wire dislodgement (13,23).
Pneumothorax risk is high (24–68%) but when it occurs, it is usually asymptomatic and only 0–4.6% requires an intervention (20-22). Factors that increase the risk of pneumothorax include the presence of emphysema, localization of several targets, and a smoking index greater than 400 (20,22). Parenchymal hematoma is another common complication (5.9–21%) and this risk is increased in lesions deeper than 25 mm (20).
Kleedehn et al. compared preoperative transthoracic hookwire with MB marking for PLLs. One hundred and two patients underwent 109 localization procedures: 52 hookwire insertions and 57 MB injections. Localization success was 98% and 100%, respectively. There was however, a trend toward more frequent and severe complications in the hookwire insertion group: pneumothorax (38% vs. 25%) and perilesional hemorrhage (12% vs. 4%). Hookwire dislodgement occurred in 7 out of 52 patients (13%) without affecting the localization rate, as the nodules were localized through visualization of the parenchymal puncture sites. There was one death secondary to systemic air embolism (32).
Combined percutaneous modalities for placement of markers
Although most institutions use a single modality for PLL localization, some authors reported using combined methods to overcome the shortcoming of using one single method (23,24). Kang et al. reported a success rate of 100% by combining hookwire and lipiodol marking without significant increases in procedure time or complications (23). Brady et al. combined hookwire and CT-guided MB techniques and reported that MB was helpful in identifying the lesion intraoperatively when wire dislodgment occurred (24).
Hookwire and Tc-99m (33), Tc-99m and MB (34), and hookwire and navigational bronchoscopic-guided MB injection (35) are other combined methods described in the literature.
Bronchoscopic placement of markers to assist resection of peripheral lung lesions
With the emergence of virtual bronchoscopy (VB), electromagnetic navigational bronchoscopy (ENB) and other bronchoscopic modalities such as radial EBUS, the placement of marking and tattooing of the PPLs has been gaining popularity worldwide. Bronchoscopic placement of markers using devices such as fiducials, and dye agents (tattoos) have been used to facilitate wedge resection of PPLs. Table 3 summarizes studies using bronchoscopic placement of markers prior to MITS.

Full table
Virtual bronchoscopy is a modality that utilizes helical CT scan images and computer software to reconstruct a 3D simulation of the tracheobronchial tree. This can be manipulated in 3D space, allowing the user to navigate the airways and obtain a real-time bronchoscopic perspective (51). With this development and the aid of ultra-thin video bronchoscopes, the technical difficulties of transbronchial marking of PPLs have been ameliorated. The development of ENB has broadened the opportunity for new diagnostic and therapeutic modalities. ENB uses an electromagnetic tracking system and a position sensor during bronchoscopy to guide navigation through the bronchial tree. The information obtained is combined with previously acquired CT images to create 3-D virtual images and a real-time navigation “route” leading to the lesion of interest, making sampling and/or marking of peripheral lung lesions possible (52-55). An important advantage is that it can be performed during the same operative session as the thoracic surgery. The inefficiency of transporting the patient from one place to another, coordinating different teams and the operating room and repeated exposures to anesthetics or sedatives can also be avoided.
Bronchoscopic placement of barium
Early methods of marking using bronchoscopic assistance for thoracic surgery were somewhat laborious and time consuming. Barium has been used with a good success rate for localization. It remains in place for a prolonged period of time, allowing for safe resections, even days after the localization of PLLs (36,37,39).
In 1997, Kobayashi et al. developed CT-guided bronchoscopic barium marking for patients undergoing fluoroscopy-assisted thoracoscopic surgery (36). In 2001 Okumura et al. reported the results of marking 21 lesions in 20 patients using the same method (37). Under local anesthesia with 4% lidocaine, thin video-bronchoscopes were orally inserted followed by tracheobronchial examination. A transbronchial aspiration cytology (TBAC) needle was inserted and directed to the lesion of interest using fluoroscopic guidance. With the needle in position, the patient was transferred to the CT scanner to confirm its location and correlation with the lesion. If the needle was correctly located, 0.1 to 1.0 mL of 50% to 100% w/v barium sulfate was infused and then confirmed under fluoroscopy. The mean procedure time for the CT-guided bronchoscopic marking was 30 minutes. VATS resection, under general anesthesia, followed between 1–12 days of the barium injection with a mean operative time of 78 minutes. This method was found to be effective and reliable (100% identification rate) without the complications derived from the transcutaneous route, such as hematomas and pneumothorax. Nonetheless, it was technically difficult.
Limitations include the increased radiation exposure from using CT and fluoroscopy, transporting patients to different settings and the need for coordination between radiology, anesthesia, the operating room, as well as pulmonary physicians and surgeons. It has been stated that barium injection can induce a mild acute inflammatory reaction within hours followed by bronchopneumonia, and eventually granulomatous inflammation, which may confound the pathologic examination (56). At the same time, given the small amount of injected Barium, these complications are unlikely to occur (25).
Bronchoscopic placement of tattoos using dye agents
Indigo carmine (IC), MB and indocyanine green (ICG) have been used to mark PLLs before VATS or RATS (Figure 1). They are safe and effective and can be injected in a similar fashion (38,40,42). IC can be seen up to 3 days of injection (38), whereas MB diffuses within hours into the tissues (42). Therefore, the selection of the dye depends on the time between the localization procedure, and the surgical resection. If the localization is done immediately before resection, then, both can be used without affecting the outcomes. ICG has also been used for identification of other cancers and evaluation of lymph nodes. It can be visualized using near infrared (NIR) technology (36,39,54) in those cases where damaged lungs, anthracosis or hemorrhage make it difficult for the targeted tissue to be differentiated using MB (Figure 1B) (44,50,57).
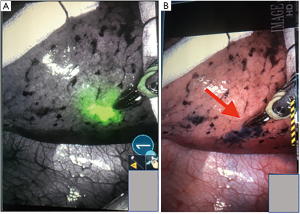
Sakamoto et al. developed a bronchoscopic dye injection technique for the localization of small pulmonary nodules prior to VATS. Six patients were included with a total of six lesions, and a mean size of 11 mm. The subsegment containing the nodule was located using a high-resolution CT. A bronchoscope was then inserted into the related subsegmental bronchus and a teflon sheath was advanced through the bronchiole beyond the tumor to the visceral pleura using CT fluoroscopy guidance. When the catheter approached the visceral pleura 0.5 mL of IC was injected. A CT was then performed to confirm the location of the injected site, which appeared as an obscure mass. The average injection time was 30 minutes and the time between the IC injection and VATS was 1–3 days. The mean operative time for VATS was not reported. None of the procedures were converted to open thoracotomy and there were no complications reported (38).
The first ENB guided intraoperative dye injection method for marking PPLs was reported by Bolton et al. They retrospectively evaluated the safety and effectiveness of ENB alone as a localization strategy for pulmonary nodules. A total of 19 patients and 19 lesions underwent ENB (SuperDimension, Minneapolis, MN, USA) localization of PPLs prior to RATS (da Vinci robotic resection) in the same operative setting. All patients received general anesthesia and ENB was performed to localize the lesion. In some instances, transbronchial needle biopsies were obtained. Once the lesion was localized, 0.5–1.0 mL of MB was injected. The catheter was then guided to the pleural surface where 0.5–1.0 mL was injected in each of two separate locations. The bronchoscope was then removed and RATS was performed. The mean nodule size was 18 mm (range: 8–40 mm) with a median marking time of 28 minutes. All lesions were identified and there were no adverse effects associated with the placement of dye for localization. No conversions to VATS or thoracotomy occurred.
Marino et al. retrospectively reviewed a total of 70 patients and 72 lesions who underwent ENB immediately followed by VATS between 2011 and 2014. Patients underwent general anesthesia and the nodules were localized using the SuperDimension navigation system. After nodule localization with ENB, the electromagnetic guide was removed and an ECW was left in place. A bronchoscopic needle was deployed with fluoroscopic guidance and used to inject 0.5 mL of MB into the nodule. If the nodule was >5 mm from the pleura surface, an additional injection of 0.5 mL was used to mark the pleural surface. The needle and the bronchoscope were removed and VATS resection was performed. The median nodule size was 8 mm and the median distance to the pleural surface was 6 mm. The mean time of the bronchoscopic marking was 28 minutes. The success rate was 97.2% with only 2 nodules not marked successfully.
ENB and endobronchial injection of dye (MB, IC, ICG) have proven to be an effective method of localization, and studies have shown the ability to identify smaller lesions, with mean localization times as low as 9.7 minutes (45). The technique is safe and effective and has the advantage of being performed in the same setting with surgical resection of PLLs (28) (Figure 2). The success rate has been reported to be between 79–100% (38,40,42,44-50). It should be noted however, that all of these studies have been retrospective, most of them have been small, and some (with a 100% success rate) were done on patients that had a high risk for malignancy. ENB-guided dye marking can, on rare occasions, be limited by dispersion of dye into the pleural space. This may occur if the needle penetrates the visceral pleura. There were no complications reported with any of the dyes used.
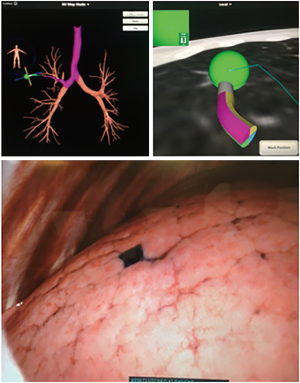
Combinations of dyes have been used to facilitate localization. MB and ICG were combined with iohexol, a radiopaque material, to facilitate the localization with fluoroscopy. The addition of this marker is useful if MB is not localized, it disperses or if ICG cannot be located under NIR (44,50).
In 2015, Anayama et al. described an animal model of localization of pulmonary nodules using ENB and a near-infrared fluorescence thoracoscope. Under general anesthesia, ICG was injected into the lung parenchyma of live porcine by transthoracic approach. After euthanasia, the geographic distribution of ICG was examined. In the same study, ENB guided ICG injection and intraoperative localization was performed. A sub centimeter pulmonary nodule was created as the objective “pulmonary nodule” by injecting 0.3 mL of 5% agar containing iopamidol into the lung parenchyma of live porcine. ENB with the cylindrical sensor (5 mm long, 0.8 mm diameter) (Aurora, Northern Digital, Waterloo, Ontario, Canada) was then used to localize the nodule and 0.1 mL of a mixture of ICG and iopamidol was injected using an Olympus transbronchial needle. The position of the injected ICG was confirmed with CT scan and was followed by VATS 6 hours later. It was found that the transbronchial application of ICG fluorescence dye can be used to localize small sized pulmonary nodules during MITS (44).
Tay et al. reviewed a retrospective cohort of consecutive patients undergoing ENB-guided pleural tattoo followed by VATS wedge resection of PPLS. Six patients underwent ENB and tattoo placement with a 25 G needle passed through an EWC under fluoroscopic guidance. When the needle was in position, 1 mL of IC was injected in 5 out of six patients and 1mL of MB was injected in one patient. The marking procedure was performed 1 day before VATS and all lesions were identified. An important feature of this study showed that the use of a radial probe EBUS was used to localize the lesions was possible (46).
Abbas et al. described a retrospective study on 51 patients and 54 nodules. Patients under general anesthesia, underwent ENB (SuperDimension) for localization. A needle was inserted through the extended working channel (EWC) and fluoroscopy was used to confirm its location. One mL of a marker solution was injected into the nodule. If the nodule was deeper than 20 mm from the pleura, a second injection of 0.5 mL was injected close to the overlying pleura. MB was used with fiducials for the first 2 patients. No added benefit from the fiducials was identified and the next 19 patients received only MB. Since MB tended to diffuse into the surrounding lung parenchyma and the added ability to monitor the injection under fluoroscopy, the marking solution was switched to 1–2 mL of a mixture of Isovue, MB and ICG injected bronchoscopically into or adjacent to the nodule. Most patients underwent RATS but only two had VATS resection of the PLLs, all immediately after the marking procedure. The tattooing mean time was 29.1 minutes and the mean operative time after marking was 212 minutes. The dye was visible in 100% of nodules with a localization success rate of 98.1%. No adverse events were identified (48).
Bronchoscopic placement of coils
Intrabronchial coil marker placement is a modality that was found to be effective when performed in combination with virtual bronchoscopy, flexible bronchoscopy and the subsequent CT confirmation of coil position. Miyoshi et al. prospectively evaluated 9 patients with 11 lesions. All patients had a CT chest performed and the images were transferred to a workstation (Alatoview, Toshiba, or Virtual Place Worksite) Reconstructed VB images were generated to one sixth generation bronchi. The VB images were used to simulate the branches of the bronchus leading to the lesions. Thin video bronchoscopes were orally inserted under local anesthesia. A transbronchial coil-feeding catheter (Boston scientific) was inserted in the bronchus and fluoroscopically guided to the lesion site. At this point a high-resolution scan was performed to assess the location. When the tip of the coil-feeding catheter had reached the lesion, a fibered platinum coil (Boston scientific) was instilled into the bronchus with CT fluoroscopic guidance. Coils used were similar to coils used for blood vessel embolism. Lung resections via VATS, as late as 30 days after the localization procedure were performed. The mean localization procedure time was 32 minutes There were no complications related to the localization procedure which had a success rate of 100% (41). Since resections can occur several days after the PLLs are marked, coordinating with the OR is more efficient. Drawbacks include the high cost of the coil, the need to transport the patient to different settings, and the higher radiation exposure when compared to some of the other bronchoscopic techniques.
Bronchoscopic placement of fiducials
Fiducial markers have been successfully used to guide stereotactic body radiotherapy. They can be placed percutaneously or with ENB (58-60). Belanger et al. performed a retrospective review of ENB cases between June 2015 and April 2017. All patients were considered for biopsy and possible fiducial marker placement. The procedures were performed under general anesthesia with endotracheal intubation or laryngeal mask airway. Airway inspection and EBUS-guided evaluation of mediastinal and hilar lymph nodes with transbronchial needle aspiration, when indicated, was performed. In cases without an established diagnosis, ENB (SPiNDrive, Veran Medical, Saint Louis, MO, USA) was subsequently performed with ENB-guided tip-tracked TBNA biopsies. If the rapid onsite evaluation of the biopsies was inconclusive then Electromagnetic-guided transthoracic needle aspiration (EMTTNA) was done. After the biopsies were obtained, fiducial marker placement was done with a tracked brush, in which the fiducial marker was loaded and then guided to the lesion or next to it and inserting it into the lung parenchyma. The fiducial markers used bronchoscopically were 0.8 mm × 3.5 mm gold seed with an attached 4 mm nitinol wire. For EMTTNA a 0.5 mm × 5 mm linear gold wire was used. Fiducial marker placement was done in 65 patients with a total of 133 markers placed. ENB was used for 131 markers and EMTTNA for only 2 of them. Migration of the marker was noted in 1 case but 2 other markers remained intact in the patient, allowing for the performance of SBRT. There were no complications related to the fiducial marker placement. In the same study, ENB was successful for PLLs biopsy with a diagnostic yield of 78% and when combined with transthoracic needle aspiration the yield increased to 87% (58). Bolton et al. reported lower rates of complications using ENB compared with CT-guided marker placement (59). In both studies fiducials were used to guide therapy and not as markers prior to MITS.
Combined percutaneous and bronchoscopic modalities for placement of markers
Sun et al. proposed a combination of ENB guided injection of MB combined with percutaneous Hookwire placement. In his case report a 50-year-old female with a 0.6 cm × 0.5 cm lesion was targeted with ENB. MB 0.6 mL was injected through an EWC. The time required to complete the procedure was 14 minutes and 25 seconds. The injection was followed by a CT chest to determine the optimal needle path, sterilization of the skin and local anesthesia. The hookwire was inserted through the chest wall and placed as close as possible to the lesion. A repeat CT chest was done to confirm the placement and identify possible complications. No complications were identified with a total time for this procedure of 16 minutes. The patient was transported to the OR and VATS was performed within one hour.
To our knowledge, there are no other reports of combined percutaneous and bronchoscopic modalities.
Other modalities
Zhao et al., used cone beam CT and ENB in a Hybrid operating room, which can provide real-time images that aid the navigation, identification and biopsy. The use of image guided percutaneous localization in the hybrid operating room has also been described (61,62).
The Brigham and Women’s Hospital iVATS evaluated 23 pulmonary lesions of size 1.30±0.38 cm which received conventional thoracoscopic surgery after the placement of two T-shaped fiducials (Kimberly-Clark, Roswell, GA, USA) under intraoperative C-arm CT. In this study, a 5-second end-inspiratory-hold 200° rotation with a 0.36 mGy/projection scanning protocol for the pre-determined field was able to identify the nodule by CBCT. Two T-bar fiducials were implanted afterwards for localization under fluoroscopic guidance according to the trajectory provided by the syngo iGuide needle guidance software. Twenty-two lesions were found to be malignant. Zhao et al. performed 19 real-time image-guided hookwire localization immediately followed by VATS resections. The mean operative time was 128 min. A total of five patients had a pneumothorax as a complication of hookwire insertion, but none required intervention (62). With this technique, the radiation exposure is not significantly higher than with other fluoroscopic techniques but the cost and complexity of the equipment maybe a challenge (61).
Lachkar et al. reported successful dye marking using VB biopsy of PPLs with radial endobronchial ultrasound (EBUS) (63). Combined radial EBUS and ENB has a diagnostic yield of 47.1% for PPLs (64). This suggests that radial EBUS can be used in combination with ENB to assist in PLLs marking before MITS. Prospective studies evaluating the efficacy and cost/benefit of these modalities are needed.
Conclusions
Pulmonary nodules have malignant potential. With the increased use of CT scans, advances in technology, and lung cancer screening guidelines, smaller and an increased number of smaller lesions are being detected. This can be challenging for the thoracic surgeon during MITS. Multiple modalities have been developed to assist in the localization of lesions, resulting in the reduction of the operating time and conversion to thoracotomy. Transthoracic marking has been popular in the past. Bronchoscopic techniques with similar efficacy and lower complication rates are attractive since they can be performed intraoperatively without major technical complications.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [Crossref] [PubMed]
- Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:7S-37S.
- Daniel TM, Kern JA, Tribble CG, et al. Thoracoscopic surgery for diseases of the lung and pleura. Effectiveness, changing indications, and limitations. Ann Surg 1993;217:566-74; discussion 574-5. [Crossref] [PubMed]
- Fang L, Wang L, Wang Y, et al. Video assisted thoracic surgery vs. thoracotomy for locally advanced lung squamous cell carcinoma after neoadjuvant chemotherapy. J Cardiothorac Surg 2018;13:128. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793-802. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Sharma A, McDermott S, Mathisen DJ, et al. Preoperative Localization of Lung Nodules With Fiducial Markers: Feasibility and Technical Considerations. Ann Thorac Surg 2017;103:1114-20. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [Crossref] [PubMed]
- Tseng YH, Lee YF, Hsieh MS, et al. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S666-71. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Li C, Liu B, Jia H, et al. Computed tomography-guided hook wire localization facilitates video-assisted thoracoscopic surgery of pulmonary ground-glass nodules. Thorac Cancer 2018;9:1145-50. [Crossref] [PubMed]
- Kang DY, Kim HK, Kim YK, et al. Needlescopy-assisted resection of pulmonary nodule after dual localisation. Eur Respir J 2011;37:13-7. [Crossref] [PubMed]
- Brady JJ, Hirsch Reilly C, Guay R, et al. Combined Hookwire and Methylene Blue Localization of Pulmonary Nodules: Analysis of 74 Patients. Innovations (Phila) 2018;13:184-9. [Crossref] [PubMed]
- Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Nomori H, Horio H. Colored collagen is a long-lasting point marker for small pulmonary nodules in thoracoscopic operations. Ann Thorac Surg 1996;61:1070-3. [Crossref] [PubMed]
- Wu TT, Chang YC, Lee JM, et al. Anaphylactic reaction to patent blue V used in preoperative computed tomography-guided dye localization of small lung nodules. J Formos Med Assoc 2016;115:288-9. [Crossref] [PubMed]
- Kleedehn M, Kim DH, Lee FT, et al. Preoperative Pulmonary Nodule Localization: A Comparison of Methylene Blue and Hookwire Techniques. AJR Am J Roentgenol 2016;207:1334-9. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Wang YZ, Boudreaux JP, Dowling A, et al. Percutaneous localisation of pulmonary nodules prior to video-assisted thoracoscopic surgery using methylene blue and TC-99. Eur J Cardiothorac Surg 2010;37:237-8. [Crossref] [PubMed]
- Sun J, Mao X, Xie F, et al. Electromagnetic navigation bronchoscopy guided injection of methylene blue combined with hookwire for preoperative localization of small pulmonary lesions in thoracoscopic surgery. J Thorac Dis 2015;7:E652-6. [PubMed]
- Kobayashi T, Kaneko M, Kondo H, et al. CT-guided bronchoscopic barium marking for resection of a fluoroscopically invisible peripheral pulmonary lesion. Jpn J Clin Oncol 1997;27:204-5. [Crossref] [PubMed]
- Okumura T, Kondo H, Suzuki K, et al. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg 2001;71:439-42. [Crossref] [PubMed]
- Sakamoto T, Takada Y, Endoh M, et al. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg 2001;72:296-7. [Crossref] [PubMed]
- Asano F, Shindoh J, Shigemitsu K, et al. Ultrathin bronchoscopic barium marking with virtual bronchoscopic navigation for fluoroscopy-assisted thoracoscopic surgery. Chest 2004;126:1687-93. [Crossref] [PubMed]
- Endo M, Kotani Y, Satouchi M, et al. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest 2004;125:1747-52. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014.4. [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg 2015;99:224-30. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Tay JH, Wallbridge PD, Larobina M, et al. Electromagnetic Navigation Bronchoscopy-directed Pleural Tattoo to Aid Surgical Resection of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2016;23:245-50. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Munoz-Largacha JA, Ebright MI, Litle VR, et al. Electromagnetic navigational bronchoscopy with dye marking for identification of small peripheral lung nodules during minimally invasive surgical resection. J Thorac Dis 2017;9:802-8. [Crossref] [PubMed]
- Ng CSH, Zhao Z, Long H, et al. Electromagnetic Navigation Bronchoscopy Triple Contrast Dye Marking for Lung Nodule Localization. Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [PubMed]
- Vining DJ, Liu K, Choplin RH, et al. Virtual bronchoscopy. Relationships of virtual reality endobronchial simulations to actual bronchoscopic findings. Chest 1996;109:549-53. [Crossref] [PubMed]
- Brady J, Chang R, Rudoler S. Electromagnetic Navigational Bronchoscopy: New Modality, New Diagnosis. Chest 2014;146:780A. [Crossref]
- Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017;17:59. [Crossref] [PubMed]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: A descriptive analysis. J Thorac Dis 2012;4:173-85. [PubMed]
- Mahajan AK, Patel SB, Hogarth DK. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnosing peripheral lung lesions unreachable by conventional bronchoscopy. Chest 2008;134:98P. [Crossref]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: Experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Chiu CH, Chao YK, Liu YH, et al. Clinical use of near-infrared fluorescence imaging with indocyanine green in thoracic surgery: a literature review. J Thorac Dis 2016;8:S744-8. [Crossref] [PubMed]
- Belanger AR, Burks AC, Chambers DM, et al. Peripheral Lung Nodule Diagnosis and Fiducial Marker Placement Using a Novel Tip-Tracked Electromagnetic Navigation Bronchoscopy System. J Bronchology Interv Pulmonol 2019;26:41-8. [Crossref] [PubMed]
- Bolton WD, Richey J, Ben-Or S, et al. Electromagnetic Navigational Bronchoscopy: A Safe and Effective Method for Fiducial Marker Placement in Lung Cancer Patients. Am Surg 2015;81:659-62. [PubMed]
- Rose M. Bronchoscopic delivery of lipiodol as a fiducial marker in lung tumors before radiotherapy. J Thorac Oncol 2014;9:1579-83. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Yu PS, et al. Image-guided localization of small lung nodules in video-assisted thoracic surgery. J Thorac Dis 2016;8:S731-7. [Crossref] [PubMed]
- Zhao ZR, Lau RWH, Ng CSH. Hybrid Theater and Uniportal Video-Assisted Thoracic Surgery: The Perfect Match for Lung Nodule Localization. Thorac Surg Clin 2017;27:347-55. [Crossref] [PubMed]
- Lachkar S, Baste JM, Thiberville L, et al. Pleural Dye Marking Using Radial Endobronchial Ultrasound and Virtual Bronchoscopy before Sublobar Pulmonary Resection for Small Peripheral Nodules. Respiration 2018;95:354-61. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]


