
Genomic alterations and survival in young patients aged under 40 years with completely resected non-small cell lung cancer
Introduction
Lung cancer is the leading cause of neoplasm-related death in China (1). At diagnosis, most patients are a median age of over 60 years. Less than 2% of patients are under 45 years of age in Caucasian populations and 5% in China (2,3). However, with the rising incidence in lung cancer, an increasing number of patients are younger at initial presentation.
An examination of genomic alterations is often required for many carcinomas before initiating systemic treatment for older patients. For example, breast cancer is associated with a higher frequency of BRCA mutations in young patients, and colon cancer in young patients has a higher rate of microsatellite instability compared with older patients (4,5). Despite the lower frequency of lung cancer in younger people, studies of genomic alterations are lacking for non-small cell lung cancer (NSCLC). Furthermore, most young patients with lung cancer are at an advanced stage at first diagnosis (6). The clinicopathological characteristics and genomic alterations are largely unknown among patients who undergo surgery. A larger population study is required for describing genomic alterations and outcomes of lung cancer in young patients. Meanwhile, clinical presentations and prognoses for lung cancer have remained scant for young patients. Previous studies have provided diverging results indicating whether younger patients with lung cancer had better or worse survival compared with older patients (6,7). It might explain the drawbacks of various retrospective studies with small sample sizes (8,9).
The objective of our study was to examine the genomic alterations and prognosis in young patients so as to guide clinical therapeutics.
Methods
Patient selection
A total of 736 consecutive patients undergoing complete resection were screened between 2011 and 2015 at our hospital. These patients had lung adenocarcinoma (n=430), squamous cell carcinoma (SCC, n=210) and other histologies (n=96), of which patients with other histologies were excluded from our study. The study protocol was approved by our institutional Ethics Committee (IRB-2015-49), and written informed consent was obtained from each participant.
Gene analysis
Microscopy was used to ensure that the tissues analyzed contained >20% tumor cells. Genomic DNA or RNA was extracted from tumor tissues according to standard protocols (RNeasy Mini Kit & QiAamp DNA Mini Kit, Qiagen, Hilden, Germany). Briefly, isolated RNA samples were used for reverse transcription into cDNA using the Revert Aid First Strand cDNA synthesis kit (Fermentas, St Leon-Rot, Germany). Either genomic DNA or cDNA was used for PCR amplification and sequencing. EGFR, HER2, KRAS, NRAS, BRAF and PIK3CA were PCR-amplified with genomic DNA. Cycle sequencing of purified PCR products was performed with PCR primers using the commercially available ADx mutation detection kit (Amoy, Xiamen, China).
ALK, ROS1 and RET fusion mRNAs were detected by PCR with a fusion gene detection kit (Amory, Xiamen, China). Briefly, total RNA was extracted with the QiagenRNeasy FFPE Kit, and mRNA was reverse-transcribed into cDNA for 1 h at 42 °C. β-actin was used as an internal control. The RT-PCR conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 95 °C for 25 s, 64 °C for 20 s and 72 °C for 20 s to ensure specificity; and 31 cycles at 93 °C for 25 s, 60 °C for 35 s and 72 °C for 20 s. The detailed procedure has been described previously (10).
Statistical analysis
χ2 tests were used to test the differences between younger and older patients for categorical variables. Recurrence-free survival (RFS) referred to the time from surgery to recurrence or metastasis. Overall survival (OS) was defined as the time from the date of pathological diagnosis to the date of death from any cause. Survivors at the last follow-up time were censored. A survival comparison was estimated with the log-rank test and survival analysis was performed with the Kaplan-Meier method. Statistical analysis was performed with SPSS 18 software (Chicago, IL, USA). P<0.05 was deemed as statistically significant. The median follow-up was 56 (16.5–69) months and the last follow-up date was December 31, 2017.
Results
Patient characteristics
A total of 430 patients had histologically confirmed adenocarcinoma and 210 patients had SCC, all of whom underwent complete tumor resection. The stages at diagnosis were I (n=247), II (n=115) and III (n=278). There were 351 males and 289 females with a median age of 59 [20–85] years. Our cohort had 427 never-smokers and 213 previous/current-smokers. Patient characteristics are summarized in Table 1. In total, 54 patients were under 40 years of age (the “younger” group) and 586 were over 40 years (the “older” group). Among the 54 younger patients, there were 11 SCC cases and 43 lung adenocarcinoma cases. Compared with having an advanced age, younger age was associated with an increased likelihood of being a never-smoker (P=0.04) or female (P=0.058). More patients had a histology of lung adenocarcinoma among the younger patients (P=0.04). The comparative details between the younger and older groups are listed in Table 1.
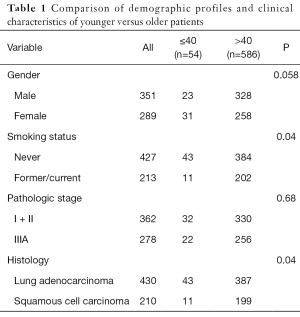
Full table
Gene alterations and comparison
Among 640 patients, 358 patients had identified genomic alterations (55.9%). Their genotypes included EGFR mutations (n=223, 34.8%), followed by KRAS (n=28, 4.4%), PIK3CA (n=24, 3.8%), EML4-ALK rearrangements (n=17, 2.7%), HER2 (n=12, 1.9%), ROS1 (n=11, 1.7%), RET (n=8, 1.3%), BRAF (n=2, 0.3%), NRAS (n=1, 0.2%) and coexisting mutations in other genes (n=24, 3.8%). The frequencies of genomic alterations in the younger and older groups were 68.5% and 54.8%, respectively (P=0.05). There was a frequency difference between the younger and older groups in fusion genes (22.2% vs. 4.1%, P<0.001) but not in gene mutations (46.3% vs. 45.6%, P=0.92).
Among 430 patients with lung adenocarcinoma, 43 patients were in the younger group and 397 were in the older group. The overall frequency of gene alterations in these groups were 76.7% and 71.8%, respectively (P=0.49). There were more patients with fusions genes in the younger than the older group (23.3% vs. 5.9%, P=0.0001). However, no inter-group difference existed in gene mutations (53.4% vs. 60.2%, P=0.39; Table 2) (Figure 1A).
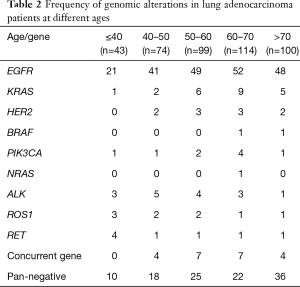
Full table
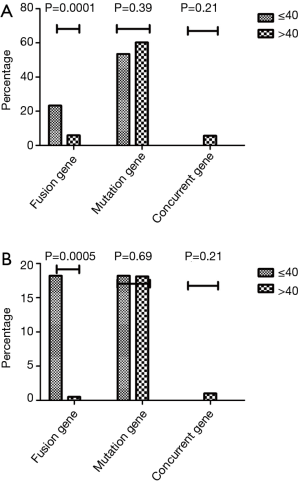
Among 210 SCC patients, 11 were younger, while 199 were older (n=199). There were no inter-group differences in the frequency of gene alterations (36.4% vs. 16.7%, P=0.21). However, alterations in fusion genes were more frequent in the younger than in the older group (18.2% vs. 0.5%, P=0.0005; Table 3) (Figure 1B).
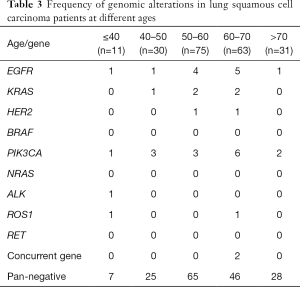
Full table
Treatment and survival comparisons
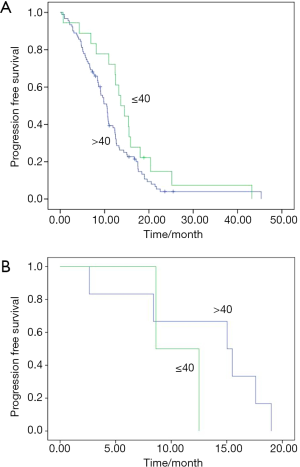
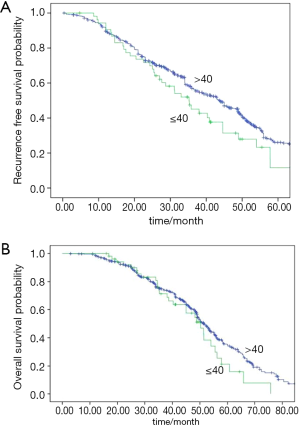
A total of 410 cases recurred or became metastatic after complete resection, including 41 younger cases and 379 older cases. Among 379 older patients, 97 of 201 with positive gene alterations received targeted treatment, including EGFR-TKIs (n=91) and ALK inhibitors (n=6). Among 41 younger patients, 20 of 31 with gene mutations received targeted treatment, including EGFR-TKIs (n=18) and ALK inhibitors (n=2). Compared with the older group, there was a trend towards a larger percentage of younger patients on targeted treatments than older patients (67.1% vs. 48.3%, P=0.09). No difference in progression-free survival (PFS) existed between younger and older cases, either in EGFR-TKIs or ALK inhibitor treatment (P=0.11 and 0.29, respectively, Figure 2A,B). The values of median RFS and OS for all patients were 43.0 (95% CI: 39.4–46.6) and 51.3 (95% CI: 49.3–53.2) months, respectively. There was a trend of shorter RFS in younger than older patients (35.2 vs. 43.8 months, P=0.050; Figure 3A). A multivariate Cox’s regression model of RFS was constructed from the variables gender, stage, smoking and age. Stage qualified as independent prognostic factor in patients (P=0.03). However, no inter-group difference existed in OS (50.2 vs. 51.4 months, P=0.112; Figure 3B). A trend towards longer OS was found in gene alteration-positive patients than those who were negative for these mutations (53.7 vs. 49.1 months, P=0.088).
Discussion
In the present study, all NSCLC patients underwent complete resection. From our data, we found that there was a significantly greater frequency of gene abnormalities in younger than older patients, especially in fusion genes. RFS was shorter among younger patients than older cases, and a trend of worse OS in younger patients. To the best of our knowledge, our study contains the largest number of patients for detecting multi-gene abnormalities in younger NSCLC patients from an Asian population.
The correlation between age and gene abnormalities is controversial. According to several Asian studies, there is a trend of higher prevalence of older patients harboring EGFR mutations (11,12). However, other studies failed to support this finding (13,14). One study by Sacher et al. demonstrated that a greater proportion of younger patients had EGFR mutation in a Caucasian population (15). Racial differences might contribute to the diverging results of these studies. It is well-known that KRAS mutations are predominant in Caucasian patients, while EGFR is the most frequently mutated gene in Asian populations. In the present study, no EGFR mutation differences were found in our cohort. According to previous studies, the frequency of ALK fusion genes was greater in younger patients and a similar trend existed for ROS1-positive patients (16,17). Furthermore, the frequency of ALK/ROS1/RET fusions was much higher in younger than older patients. This agrees with the results of previous studies.
Most previous genetic studies have focused on lung adenocarcinoma. Non-adenocarcinoma patients have rarely been examined. In a study by Sacher et al., 2,237 patients of different ages were recruited to detect gene abnormalities but only 29 were SCC patients (15). In the present study, no difference in gene abnormalities was found between younger and older SCC patients, except for fusion genes, among 210 patients with SCC. Thus, patients with fusion genes tended to be younger, regardless of histology.
It has remained controversial whether age should be used as a predictor for survival in patients with carcinomas (16-20). Breast cancer in younger patients has a more aggressive disease biology and higher mortality (21,22). Colon cancer in young patients was also associated with more aggressive disease biology (23,24). In a study by Sacher et al., the youngest patients exhibited the worst survival compared with other age categories (15). Our study revealed a general trend of shorter RFS in younger than older cases. However, there was no inter-group survival difference. The reason might lie in an imbalance of treatment after recurrence or metastasis. In our study, more patients received targeted treatment in younger compared with older patients, so that could extend the survival time of younger patients after recurrence.
Our study has some inherent limitations. Our primary data were acquired retrospectively and there were a relatively small number of younger patients. Second, our subjects were recruited from a single institution. Last, not all patients with gene alternations received targeted treatment, and there was a significant bias of targeted treatment between the younger and older patients. As a result, the survival analysis could be biased.
In summary, there were some differences in the gene abnormalities detected in younger NSCLC patients. Additionally, younger patients tended to have more aggressive carcinomas than older patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional Ethics Committee (IRB-2015-49) and written informed consent was obtained from all patients.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Jemal A, Ma J, Rosenberg PS, et al. Increasing lung cancer death rates among young women in southern and midwestern states. J Clin Oncol 2012;30:2739-44. [Crossref] [PubMed]
- Zhang J, Chen SF, Zhen Y, et al. Multicenter Analysis of Lung Cancer Patients Younger Than 45 Years in Shanghai. Cancer 2010;116:3656-62. [Crossref] [PubMed]
- Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: poor survival despite intensive treatment. PLoS One 2009;4:e7695. [Crossref] [PubMed]
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69-77. [Crossref] [PubMed]
- Hsu CL, Chen KY, Shih JY, et al. Advanced non-small cell lung cancer in patients aged 45 years or younger: outcomes and prognostic factors. BMC Cancer 2012;12:241. [Crossref] [PubMed]
- Nagashima O, Ohashi R, Yoshioka Y, et al. High prevalence of gene abnormalities in young patients with lung cancer. J Thorac Dis 2013;5:27-30. [PubMed]
- Larrieu AJ, Jamieson WR, Nelems JM, et al. Carcinoma of the lung in patients under 40 years of age. Am J Surg 1985;149:602-5. [Crossref] [PubMed]
- Jubelirer SJ, Wilson RA. Lung cancer in patients younger than 40 years of age. Cancer 1991;67:1436-8. [Crossref] [PubMed]
- Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol. 2015;10:778-83. [Crossref] [PubMed]
- Ueno T, Toyooka S, Suda K, et al. Impact of age on epidermal growth factor receptor mutation in lung cancer. Lung Cancer 2012;78:207-11. [Crossref] [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [Crossref] [PubMed]
- Hong S, Fang W, Hu Z, et al. A Large-scale Cross-sectional Study of ALK Rearrangements and EGFR Mutations in Non-small-cell Lung Cancer in Chinese Han Population. Sci Rep 2014;4:7268. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Sacher AG, Dahlberg SE, Heng J, et al. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:313-20. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Skarin AT, Herbst RS, Leong TL, et al. Lung cancer in patients under age 40. Lung Cancer 2001;32:255-64. [Crossref] [PubMed]
- Mauri D, Pentheroudakis G, Bafaloukos D, et al. Non-small cell lung cancer in the young: a retrospective analysis of diagnosis, management and outcome data. Anticancer Res 2006;26:3175-81. [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive Characteristics of Non-small Cell Lung Cancer (NSCLC) in the Young, A Surveillance, Epidemiology, and End Results (SEER) Analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Ramalingam S, Pawlish K, Gadgeel S, et al. Lung cancer in young patients: analysis of a surveillance, epidemiology, and end results database. J Clin Oncol 1998;16:651-7. [Crossref] [PubMed]
- Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol 2010;21:1974-81. [Crossref] [PubMed]
- Azim HA Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014;16:427. [Crossref] [PubMed]
- Liu B, Farrington SM, Petersen GM, et al. Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med 1995;1:348-52. [Crossref] [PubMed]
- Wang MJ, Ping J, Li Y, et al. The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep 2015;5:10645. [Crossref] [PubMed]


