Clinical manifestations of Wilson disease in organs other than the liver and brain
Introduction
Copper is an element that is essential for the function of a variety of enzymes that participate in many physiological pathways (1-3). However, in Wilson disease (WD), because of mutations in the copper-transporting ATPase, ATP7B gene, plasma levels of toxic non-ceruloplasmin-bound copper are elevated. Deposits of toxic copper accumulate in different organs. Although the most common presentation of WD involves hepatic and neurologic disturbances, there are other less common signs that are also important for diagnosis and treatment (1-3). Very high copper content was found in the kidney, heart and osseous matter of WD patients compared to healthy individuals (4). Symptoms may result from direct toxicity of copper, oxidative stress, and as a consequence of liver failure or anti-copper treatment (1-3). Some of the symptoms are mild or subclinical and are often dismissed. However, awareness of possible WD manifestations, other than hepatic or neurological features, is important for diagnosis WD patients.
In this paper, we describe less typical but important clinical features that may aid in the diagnosis and treatment monitoring of WD.
Ophthalmologic signs
The most typical ophthalmologic sign is the Kayser-Fleischer (K-F) (Figure 1A) ring that is formed as a result of copper deposition in Descemet’s membrane in the cornea (1-6). K-F rings usually occur in both eyes, range in color from greenish gold to brown, and are present in almost all neurologic WD patients, 50% of hepatic patients and 20–30% of presymptomatic (1,2). The standard method for K-F rings evaluation is a slit-lamp examination. In patients in advanced stages of disease, it may be seen by naked eye. Recently, it has been shown that anterior-segment optical coherence tomography (AS-OCT) may serve as an accurate objective method to assess the presence of copper deposits that form K-F rings (Figure 1B) (7,8). A most recent study analyzed 52 WD patients and a control group using in vivo confocal microscopy (IVCM). The results showed that IVCM may also be used in objective identification of K-F rings (9). After anti-copper therapy K-F rings usually disappear (1-6).
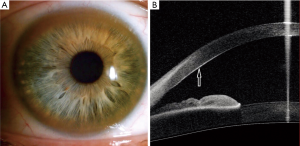
Sunflower cataract is a rare but also characteristic sign, where copper deposits are situated under the lens capsule. Notably, sunflower cataract is not associated with impaired vision (10,11). Resolution of this ophthalmologic change after anti-copper treatment has also been reported (11).
Recent studies have shown that also retina and optic nerve may be affected (12,13). Reduced thickness of retinal nerve fiber was observed in WD patients, especially in those with marked brain damage and this correlated with unified WD rating scale (UWDRS). Thus, degeneration of the retina in WD may serve as a marker of neurodegeneration and correlate with the degree of impairment of the nervous system (14).
Osteoarticular disturbances
Osteoarticular disturbances are common in WD patients, and include osteopenia, osteoporosis, skeletal abnormalities, and arthropathy. Bone demineralization may lead to osteopenia and osteoporosis and in one study, osteoporosis was detected in 43% of analyzed WD patients (15). The prevalence of fracture was reported in 7% to 14% of WD patients (16-18). Disturbances of the skeleton, like congenital scoliosis, bone demineralization, subchondral fragmented bones and cysts, have been reported in WD (14,19-22). Joint disturbances may possibly be caused by copper deposition (23,24). The most commonly affected joints are knees and wrists (4). A Chinese study reported eight patients with osseomuscular WD characterized by onset with arthropathy without initial typical neurologic or hepatic symptoms (25). WD patients may also suffer from drug-related arthritis caused by d-penicillamine (DPA) treatment (4).
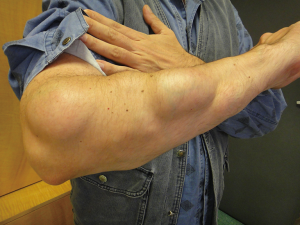
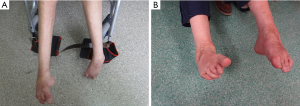
Alternatively, distortion of the joints may result from the dystonic posture of hands or feet as observed in a WD patient from our center affected with foot dystonia (Figure 2A); surgical reconstruction improved lower-extremity function (Figure 2B).
Cardiac disturbances
Cardiac abnormalities like arrhythmia, cardiomyopathy, and autonomic disturbances are also observed in WD patients. However, these disturbances are rather mild (26,27). However, a recent study in a large group of WD patients revealed an increased risk of atrial fibrillation (AF) and heart failure (HF) (28). The risk of AF was 29% higher and the risk of HF was 55% higher than in the large group of non-WD patients after adjusting for age and risk factors.
Cardiologic disturbances may be the consequence of myocardial copper deposition as well as oxidative stress as a toxic effect of elevated levels of non-ceruloplasmin-bound copper (27,29).
Electrocardiographic abnormalities presented in about 30% of WD patients include atrophic left ventricle, hypertrophy of left and right ventricles, early depolarization, sinus tachycardia, prolonged p-wave dispersion (26,30,31). In 125 WD patients in our center without previous cardiologic problems who underwent electrocardiographic and echocardiographic study, cardiac disturbances manifested mainly as slight hypertrophy with disturbed diastolic function of left ventricle. An electrocardiographic study showed that the QRS complex is wider in WD than in the control group (32).
In another study, subclinical dysautonomia detected only with electrophysiologic testing was observed in 26% of patients (33). Furthermore, clinically overt postural hypotension was observed in 10% of examined patients. Autonomic dysfunction was observed more often in patients with the neurologic form of WD than non-neurologic.
Renal involvement
Excess toxic non-ceruloplasmin-bound copper is excreted in urine (1,4,34); however, it is not correlated with renal impairment in WD patients. Renal disturbances are overlooked in many cases due to mild manifestation. Impairment of renal tubules may cause acidosis or aminoaciduria, and occurrence of urinary calculus was reported (3,34,35). High levels of calcium in the urine result from reabsorption impairment of the distal part of renal tubules and released calcium from bones (35-37). The consequence of hypercalciuria is urinary calculus. Renal stones were reported in about 16% of patients with WD (38).
Elevated levels of blood urea nitrogen, creatinine, and uric acid were measured in WD patients. The blood urea and creatinine blood concentration occurred more frequently in patients with neurological feature compared to other groups. The authors suggested that it reflects copper deposition in the kidneys (39). Hepatorenal syndrome may occur after diuretic therapy that is common in liver failure and it is diagnosed in the absence of primary renal disease (40).
DPA therapy may also result in kidney impairment, presenting as proteinuria or hematuria (41-44). Proteinuria may resolve after treatment withdrawal (41). In our group of patients with WD, proteinuria was observed in approximately 7% of patients treated with DPA (43). Thus, routine renal testing in patients treated with DPA is necessary (1,2). In WD patients treated with zinc salts, renal impairment was not detected (43,44).
Endocrine system disturbances
Endocrinological disturbances include recurrent abortions, infertility, growth disruption, and parathyroid failure (1,45-50).
Gonadal dysfunction in WD patients may result from chronic liver disease (48). Women with symptomatic WD often have amenorrhea, which may be the first symptom before occurrence of hepatic or neurological signs. Impaired procreation capability was observed 46 WD patients, and the risk of occurrence during pregnancy was higher than in general population (49). In female WD patients recurrent abortions are more frequent in untreated patients. Teratogenicity was not reported among patients taking zinc sulphate or DPA (46-49).
Other rare endocrinological disturbances include hypoparathyroidism (1) that may result from copper deposition in the parathyroid glands. A WD patient diagnosed with growth hormone deficiency has also been reported (50).
Hematologic manifestations
Hematological disturbances include acute hemolytic anemia, leucopenia, anemia, low platelet count, coagulation parameters dysfunction that result from disturbed synthesis in the liver (1,51).
Acute Coombs-negative hemolytic anemia may precede other symptoms (33). In one study, about 7% of WD patients at admission had hemolytic anemia (52). In patients with severe liver failure, marked hemolysis may result from increased non-ceruloplasmin-bound copper released from impaired hepatocytes (53,54).
Leukopenia and thrombocytopenia in WD patients may result from hypersplenism. Notably, to intensive decoppering treatment may lead to copper deficiency that presents with pancytopenia (3,55,56). We reported three patients with WD who developed copper deficiency with leukopenia during 5–16 years after zinc sulphate treatment (57).
Lipomas
Subcutaneous lipomas are often observed in patients with WD (Figure 3). In a study of 80 patients, lipomas were detected in 21 patients (26%), most often as multiple lipomas localized mainly on the trunk and extremities (58). The reason for the high frequency of lipomas in WD is unknown. One study suggests a relationship between lipomas and the ATP7B gene (58).
Skin changes
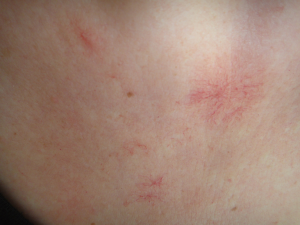
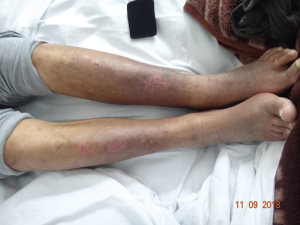
Dermatologic signs in WD may result from liver cirrhosis, such as vascular spiders (Figure 4) and palmar erythema, but other cutaneous findings may be observed during the course of the disease, for example, hyperpigmentation of the legs (Figure 5), azure lunulae of the nails, anetoderma, xerosis, acantosis nigricans, dermatomyositis (59-61). In 37 pediatric WD patients, the most common dermatologic manifestation was xerosis, which was observed in about 46% of patients (61).
Skin changes may also occur in 20–33% of patients as complications after DPA treatment (62). DPA may inhibit collagen synthesis (63) and mainly degenerative dermatoses are observed like cutis laxa, anetoderma caused with focal loss of elastic tissue, pseudoxanthoma elasticum, elastosis perforans serpiginosa (59,61,62).
Conclusions
The most common presentation of WD involves hepatic and neurological disturbances. However, deposits of toxic copper accumulate in other organs and may cause tissue injury. Extra-hepatic/neurologic signs and symptoms of WD may be a consequence of liver failure and may also be due to decoppering treatment. Other possible WD manifestations are significant in the diagnostic approach and treatment monitoring.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: All financial involvement (e.g., employment, consultancies, honoraria, stock ownership or options, grants, patents received or pending, royalties) with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the submitted publication have been completely disclosed.
References
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s Disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- Członkowska A, Litwin T, Dusek P, et al. Wilson Disease. Nat Rev Dis Primers 2018;4:21. [Crossref] [PubMed]
- Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology 2008;47:2089-111. [Crossref] [PubMed]
- Scheinberg IH, Sternlieb I. Wilson’s disease. Philadelphia: W.B. Saunders Company, 1984.
- Uzman LL, Jakus MA. The Kayser-Fleicher ring. A histochemical and electron microscope study. Neurology 1957;7:341-55. [Crossref] [PubMed]
- Wiebers DO, Hollenhorst RW, Goldstein NP. The ophthalmologic manifestations of Wilson’s disease. Mayo Clin Proc 1977;52:409-16. [PubMed]
- Sridhar MS. Advantages of Anterior Segment Optical Coherence Tomography Evaluation of the Kayser-Fleischer Ring in Wilson Disease. Cornea 2017;36:343-6. [PubMed]
- Broniek-Kowalik K, Dzieżyc K, Litwin T. Anterior segment optical coherence tomography (AS-OCT) as a new method of detecting copper deposits forming the Kayser-Fleischer ring in patients with Wilson disease. Acta Ophthalmologica 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Zhao T, Fang Z, Tian J, et al. Imaging Kayser-Fleischer Ring in Wilson Disease Using In Vivo Confocal Microscopy. Cornea 2019;38:332-7. [Crossref] [PubMed]
- Pfeiffer R. Wilson’s disease. Semin Neurol 2007;27:123-32. [Crossref] [PubMed]
- Litwin T, Langwińska-Wośko E, Dziezyc K, et al. Sunflower cataract: do not forget Wilson’s disease. Pract Neurol 2015;15:385-6. [Crossref] [PubMed]
- Albrecht P, Muller AK, Ringelstein M, et al. Retinal neurodegeneration in Wilson’s disease revealed by spectral domain optical coherence tomography. PLoS One 2012;7:e49825. [Crossref] [PubMed]
- Langwińska-Wośko E, Litwin T, Szulborski K, et al. Optical coherence tomography and electrophysiology of retinal and visual pathways in Wilson’s disease. Metab Brain Dis 2016;31:405-15. [Crossref] [PubMed]
- Langwińska-Wośko E, Kitwin T, Dziezyc K, et al. Optical coherence tomography as a marker of neurodegeneration in patients with Wilson’s disease. Acta Neurol Belg 2017;117:867-71. [Crossref] [PubMed]
- Li Z, Yu X, Shen J, et al. Congenital scoliosis in Wilson’s disease: case report and review of the literature. BMC Surg 2014;14:71. [Crossref] [PubMed]
- Hegedus D, Ferencz V, Lakatos PL, et al. Decreased bone density, elevated serum osteoprotegerin, and beta-cross-laps in Wilson disease. J Bone Miner Res 2002;17:1961-7. [Crossref] [PubMed]
- Xie YZ, Zhang XZ, Xu XH, et al. Radiologic study of 42 cases of Wilson disease. Skeletal Radiol 1985;13:114-9. [Crossref] [PubMed]
- Quemeneur AS, Trocello JM, Ea HK, et al. Bone status and fractures in 85 adults with Wilson's disease. Osteoporos Int 2014;25:2573-80. [Crossref] [PubMed]
- Warnock CG. Hepatolenticular degeneration; Wilson's disease; a report of five cases, with commentary. Ulster Med J 1952;21:155-71. [PubMed]
- Feller ER, Schumacher HR. Osteoarticular changes in Wilson’s disease. Arthritis Rheum 1972;15:259-66. [Crossref] [PubMed]
- Kaklamanis P, Spengos M. Osteoarticular changes and synovial biopsy findings in Wilson’s disease. Ann Rheum Dis 1973;32:422-7. [Crossref] [PubMed]
- Golding DN, Walshe JM. Arthropathy of Wilson’s disease. Study of clinical and radiological features in 32 patients. Ann Rheum Dis 1977;36:99-111. [Crossref] [PubMed]
- Canelas HM, Carvalho N, Scaff M, et al. Osteoarthropathy of hepatolenticular degeneration. Acta Neurol Scand 1978;57:481-7. [Crossref] [PubMed]
- Menerey KA, Eider W, Brewer GJ, et al. The arthropathy of Wilson’s disease: clinical and pathological features. J Rheumatol 1988;15:331-7. [PubMed]
- Yu H, Xie JJ, Chen YC, et al. Clinical features and outcome in patients with osseomuscular type of Wilson’s disease. BMC Neurol 2017;17:34. [Crossref] [PubMed]
- Kuan P. Cardiac Wilson’s disease. Chest 1987;91:579-83. [Crossref] [PubMed]
- Hlubocká Z, Marecek Z, Linhart A, et al. Cardiac involvement in Wilson disease. J Inherit Metab Dis 2002;25:269-77. [Crossref] [PubMed]
- Grandis DJ, Nah G, Whitman IR, et al. Wilson’s disease and cardiac myopathy. Am J Cardiol. 2017;120:2056-60. [Crossref] [PubMed]
- Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003;189:147-63. [Crossref] [PubMed]
- Turhan H, Yetkin E, Atak R, et al. Increased p-wave duration and p-wave dispersion in patients with aortic stenosis. Ann Noninvasive Electrocardiol 2003;8:18-21. [Crossref] [PubMed]
- Meenakshi-Sundaram S, Sinha S, Rao M, et al. Cardiac Involvement in Wilson’s Disease – An Electrocardiographic Observation. J Assoc Physicians India 2004;52:294-6. [PubMed]
- Buksińska-Lisik M, Litwin T, Pasierski T, et al. Cardiac assessment in Wilson’s disease patients based on electrocardiography and echocardiography examination. Arch Med Sci 2017. Available online: https://doi.org/ [Crossref]
- Meenakshi-Sundaram S, Taly AB, Kamath V, et al. Autonomic dysfunction in Wilson’s disease – a clinical and electrophysiological study. Clin Auton Res 2002;12:185-9. [Crossref] [PubMed]
- Hoogenraad TU. Wilson’s disease. In: Major Problems in Neurology, vol. 30. London: W.B. Saunders Company, 1996.
- Bearn AG, Yü TF, Gutman AB. Renal function in Wilson's Disease. J Clin Invest 1957;36:1107-14. [Crossref] [PubMed]
- Azizi E, Eshel G, Aladjem M. Hypercalciuria and nephrolithiasis as a presenting sign in Wilson disease. Eur J Pediatr 1989;148:548-9. [Crossref] [PubMed]
- Wiebers DO, Wilson DM, McLeod RA, et al. Renal stones in Wilson's disease. Am J Med 1979;67:249-54. [Crossref] [PubMed]
- Di Stefano V, Lionetti E, Rotolo N, et al. Hypercalciuria and Nephrocalcinosis as Early Feature of Wilson Disease Onset: Description of a Pediatric Case and Literature Review. Hepat Mon 2012;12:e6233. [Crossref] [PubMed]
- Wang H, Zhou Z, Hu J, et al. Renal impairment in different phenotypes of Wilson disease. Neurol Sci 2015;36:2111-5. [Crossref] [PubMed]
- Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007;56:1310-8. [PubMed]
- Davison AM, Day AT, Golding JR, et al. Effect of penicillamine on the kidney. Proc R Soc Med 1977;70:109-13. [PubMed]
- Hall CL, Jawad S, Harrison PR, et al. Natural course of penicillamine nephropathy: a long term study of 33 patients. Br Med J (Clin Res Ed) 1988;296:1083-6. [Crossref] [PubMed]
- Członkowska A, Litwin T, Karliński M, et al. D-penicillamine versus zinc sulfate as first-line therapy for Wilson's disease. Eur J Neurol 2014;21:599-606. [Crossref] [PubMed]
- Merle U, Schaefer M, Ferenci P, et al. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut 2007;56:115-20. [Crossref] [PubMed]
- Frydman M, Kauschansky A, Bonne-Tamir B, et al. Assessment of the hypothalamic-pituitary-testicular function in male patients with Wilson's disease. J Androl 1991;12:180-4. [PubMed]
- Sinha S, Taly AB, Prashanth LK, et al. Successful pregnancies and abortions in symptomatic and asymptomatic Wilson’s disease. J Neurol Sci 2004;217:37-40. [Crossref] [PubMed]
- Leggio L, Gasbarrini G, Addolorato G. Wilson’s disease. Lancet 2007;369:902. [Crossref] [PubMed]
- Karagiannis A, Harsoulis F. Gonadal dysfunction in systemic diseases. Eur J Endocrinol 2005;152:501-13. [Crossref] [PubMed]
- Tarnacka B, Rodo M, Cichy S, et al. Procreation ability in Wilson’s disease. Acta Neurol Scand 2000;101:395-8. [Crossref] [PubMed]
- Koch A, Dörr HG, Gerlin S, et al. Effect of Growth Hormone on IGF-I Levels in a Patient with Growth Hormone Deficiency and Wilson Disease. Horm Res 1995;44:40-4. [Crossref] [PubMed]
- Schaefer M, Weber L, Gotthardt D, et al. Coagulation Parameters in Wilson Disease. J Gastrointestin Liver Dis 2015;24:183-8. [PubMed]
- Saito T. Presenting symptoms and natural history of Wilson disease. Eur J Pediatr 1987;146:261-5. [Crossref] [PubMed]
- Seth B, Gavhane J, Revathi N, et al. Haemolytic anaemia – Initial presentation of Wilson Disease. Indian J Basic Applied Med Res 2015;4:498-500.
- Czlonkowska A. A study of haemolysis in Wilson’s disease. J Neurol Sci 1972;16:303-14. [Crossref] [PubMed]
- Yarze JC, Martin P, Munoz SJ, et al. Wilson’s disease: current status. Am J Med 1992;92:643-54. [Crossref] [PubMed]
- Horvath J, Beris P, Giostra E, et al. Zinc-induced copper deficiency in Wilson disease. J Neurol Neurosurg Psychiatry 2010;81:1410-1. [Crossref] [PubMed]
- Dzieżyc K, Litwin T, Sobańska A, et al. Symptomatic copper deficiency in three Wilson's disease patients treated with zinc sulphate. Neurol Neurochir Pol 2014;48:214-8. [Crossref] [PubMed]
- Schaefer M, Gotthardt DN, Didion C, et al. Increased Prevalence of Subcutaneous Lipomas in Patients With Wilson Disease. J Clin Gastroenterol 2015;49:e61-3. [Crossref] [PubMed]
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology, 11th edition. London: Saunders Elsevier, 2011.
- Ivanova II, Kotzev IA, Atanassova MV, et al. Wilson’s disease in association with anetoderma. Clin J Gastroenterol 2015;8:52-6. [Crossref] [PubMed]
- Seyhan M, Erdem T, Selimoğlu MA, et al. Dermatological signs in Wilson's disease. Pediatr Int 2009;51:395-8. [Crossref] [PubMed]
- Iozumi K, Nakagawa H, Tamaki K. Penicillamine-induced degenerative dermatoses: Report of a case and brief review of such dermatoses. J Dermatol 1997;24:458-65. [Crossref] [PubMed]
- Siegel RC. Collagen cross-linking. Effect of D-penicillamine on cross-linking in vitro. J Biol Chem 1977;252:254-9. [PubMed]