
Enhancing autophagy protects platelets in immune thrombocytopenia patients
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder in which platelet autoantigens lead to immune-mediated platelet destruction and/or suppression of platelet production (1). Clinically, ITP is characterized by thrombocytopenia, skin and mucosal bleeding, and maturation arrest of bone marrow megakaryocytes. In addition, the humoral and cellular immune dysfunctions lead to platelet destruction (2). Other studies (3,4) and our studies (5,6) have indicated that platelet apoptosis increased in ITP patients. Cytotoxic T cells (CTLs) and platelet-specific autoantibodies are involved in the platelet apoptosis.
There is evidence showing that there is a close relationship between apoptosis and autophagy (7,8). Autophagy has been shown to counteract caspase-dependent apoptosis after viral infection (9-11). Furthermore, autophagy can attenuate apoptosis in a variety of pathological processes (12,13). Autophagy is a process responsible for the removal of long-lived proteins, damaged organelles and malformed proteins via lysosomes. This self-digesting mechanism is crucial for the maintenance of intracellular homeostasis, defense, and normal growth control (14,15). A recent study shows platelet autophagy in healthy subjects, as evidenced by the autophagy-related (ATG) proteins ATG5, ATG7 and microtubule-associated protein 1 light chain 3 (LC3) (16). LC3 is the most widely investigated autophagy-related protein. Generally, green fluorescent protein-LC3 (GFP-LC3) is employed to detect autophagosomes, through indirect immunofluorescence or direct fluorescence microscopy. Platelet autophagy can be adjusted through class III PtdIns3K activity and signals initiated by the inhibition of mammalian target of rapamycin (mTOR). Platelet autophagy is an important for the stability of platelet life span and physiological functions. However, whether platelet autophagy is involved in the pathogenesis of ITP is still poorly understood.
Generally, autophagy can occur in all cell types under physiological conditions (17), and it may be enhanced after starvation or during cell differentiation (18,19). PI3K/AKT/mTOR is one of the majors signaling pathways that has been widely studied in autophagy. mTOR is a key kinase and negative regulator in the PI3K/AKT/mTOR signaling pathway and can regulate cell proliferation, growth, survival, and angiogenesis under physiological conditions and in the presence of environmental stress (20). Under stress [such as starvation, hypoxia, heat, inflammation and medical or pharmacological treatment with rapamycin (RAPA)], mTOR activity is inhibited, which then induces autophagy (21). 3-methyladenine (3-MA) is a specific inhibitor of class III PtdIns3K pathway and often used to inhibit starvation- or RAPA-induced autophagy (22). In recent years, some factors (such as biological factors and chemical factors) have employed to induce autophagy in studies. In addition to the commonly used autophagy regulators [RAPA, 3-MA, chloroquine (CQ), and oxidized low density lipoprotein (ox-LDL)], we have synthesized and screened a variety of novel small-molecule compounds. One of these small-molecular compounds, ABO (6-amino-2,3-dihydro-3-hydroxymethyl-1,4-benzoxazine), was found to efficiently avert apoptosis by inducing autophagy and could serve as an autophagy enhancer (23). In our previous studies, results showed the ABO-stimulated autophagy was related to the elevation of intracellular free Ca2+ in mTOR-independent and Annexin A7 (ANXA7)-dependent manners (24).
Currently, little is known about platelet autophagy in ITP, and whether platelet autophagy can alleviate platelet destruction in ITP is still unknown. This study was to investigate the platelet autophagy in ITP patients, and explore the effects of platelet autophagy. Our study may provide evidence for the future treatment of ITP.
Methods
Patients and controls
Forty-five newly-diagnosed ITP patients (within 3 months of diagnosis) were included in this study. There were 24 females and 21 males with the median age of 39 years (range, 23–67 years; Table 1). The platelet count ranged from 3×109 to 36×109/L (median, 17×109/L; Table 1). All the patients met the diagnostic criteria for ITP (2), and did not receive any medication. Thirty-five healthy adults served as controls. There were 20 females and 15 males with the median age of 41 years (range, 20–65 years). Platelet count ranged from 107×109 to 314×109/L (median: 183×109/L).
Full table
Patients were recruited between January 2016 and December 2017 from the Department of Hematology in Qilu Hospital of Shandong University and the Second Hospital of Shandong University. Informed consent was obtained from each patient and healthy control before study which was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Medical Ethical Committee of Qilu Hospital of Shandong University and the Second Hospital of Shandong University.
Reagents and antibodies
RAPA (R8781), 3-MA (M9281), dimethyl sulfoxide (DMSO, D5879), and poly-L-lysine (PLL, P1399) were purchased from Sigma Aldrich. M199 medium was from Gibco (12340-030). ABO was synthesized as previously reported (23) and dissolved in DMSO (0.1 mol/L). CCK-8 assay was from BestBio (BB-4202). Mitochondrial membrane potential assay kit with JC-1 was from Beyotime Biotechnology.
LC3-β primary antibody (Cell Signaling Technology, 2775) was from Cell Signaling Technology. The antibodies to ACTB (β-actin, A5316) and TUBA (α-tubulin, T9026) were from Sigma Aldrich. Alexa Fluor 488- and 546-conjugated secondary antibodies for immunofluorescence staining were from Molecular Probes. The secondary antibodies for Western blotting were donkey anti-rabbit IRDye800CW (926-32213) and donkey anti-mouse IRDye680 (926-32222) from LI-COR Biosciences.
Human platelet isolation and culture
Peripheral blood was collected from ITP patients and healthy controls, anti-coagulated with sodium citrate and centrifuged at 150 ×g for 8 min to collect platelet-rich plasma (PRP). Autologous platelets were separated from PRP by centrifuging at 800 ×g for 10 min. Platelet-poor plasma (PPP) was stored for use later. Precipitate was washed twice with phosphate-buffered saline (PBS, pH =7.2), and re-suspended at 1×107 platelets/mL for cell culture.
Platelets were re-suspended and cultured in M199 medium at 1×107 platelets/well in 24-well plate with 200 nM RAPA, 3 mM 3-MA, and 50 µmol/L ABO. Blank and healthy controls were used. Cells were maintained in a humidified environment with 5% CO2 at 37 °C for 2 h (16,23).
Immunofluorescence staining of LC3
For immunofluorescence staining, platelets maintained in PLL-coated dish were fixed with pre-cooled 4% paraformaldehyde for 15 min and blocked with 3% normal goat serum for 20 min at room temperature. Then, platelets were incubated with anti-LC3-β and anti-TUBA primary antibodies at 4 °C overnight and then treated with Alexa Fluor 488- and 546-conjugated secondary antibodies (1:200) at 37 °C for 1 h. After washing thrice with PBS, samples were observed under a laser scanning confocal microscope (LSCM; Leica) with a 100×/1.4 NA oil immersion lens.
Detection of LC3 and p62 protein expression by Western blotting
After incubation, platelets were collected by centrifugation and then re-suspended in lysis buffer. Proteins were collected, quantified, and then separated by SDS-PAGE. Subsequently, these proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, IPVH00010). After blocking with 5% (w/v) non-fat milk in PBS-Tween 20 (PBST; 0.05%) for 1 h, the membrane was stained with anti-LC3-β, p62 or anti-ACTB primary antibody (1:1,000 in PBST) at 4 °C overnight. After washing in PBST thrice, the membrane was incubated with corresponding horseradish peroxidase-conjugated secondary antibody (1:5,000) for 1 h at room temperature. The immunoreactive bands were developed with an ECL Western blotting system (Thermo Fisher, 34080). ACTB was used as a loading control. The relative quantity of proteins was analyzed with ImageJ software.
Assessment of platelet viability
CCK-8 assay was performed to detect platelet viability. PRP containing 1.5×107 platelets or PPP was diluted with M199 to 100 µL in 96-well plates. After treatment with RAPA, 3-MA, or ABO at 37 °C for 2 h, 20 µL of CCK-8 was added to each well. The platelet suspension was incubated for 4 h at 37 °C, and the optical density (OD) was detected with a microplate reader (BIO-RAD, Model 680) at 450 nm. The platelet viability was calculated by subtracting the OD of PPP from that of PRP.
Detection of platelet apoptosis
Platelet apoptosis was measured using a mitochondrial membrane potential assay kit with JC-1, a marker of mitochondrial activity. In undamaged cells, mitochondria have a high mitochondrial transmembrane potential (ΔΨm). Breakdown of ΔΨm is a characteristic of early apoptosis. ΔΨm in platelets can be measured by cell-penetrating lipophilic cationic fluorochrome JC-1. Platelets (1×107) were harvested and mixed with 0.5 mL of JC-1 staining working solution. After incubation at 37 °C for 20 min, platelets were centrifuged at 2,000 ×g for 5 min and washed in JC-1 staining buffer. Platelets were analyzed on a Beckman Gallios™ Flow Cytometer (Beckman Coulter).
The platelet apoptosis was also detected with Annexin V kit. PRP was diluted with M199 to 1×107 and treated with RAPA, 3-MA, or ABO at 37 °C for 2 h. Platelets were harvested and incubated with binding buffer and 5 mL of FITC-conjugated annexin V (Invitrogen) for 15 min. Fluorescence was quantified within 1 h on a FACSCalibur (Becton Dickinson), and data were analyzed using FlowJo Software.
Statistical analysis
Statistical data are expressed as mean ± standard deviation (SD). Comparisons were done with Student’s t-test between groups. The statistical analysis was performed with SPSS version 13.0 for Windows. A value of P<0.05 was considered statistically significant.
Results
Platelet autophagy reduced in ITP patients
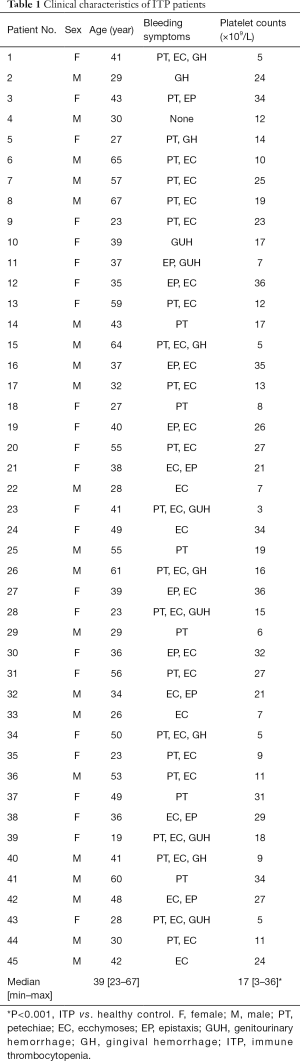
The level of platelet autophagy was compared between 20 ITP patients and 20 healthy controls. During autophagy, LC3-I is converted to LC3-II, which localizes to the phagophore and autophagosome. LC3-II accumulated in the cytoplasm indicates the presence of autophagy. Immunofluorescence staining showed LC3-positive fluorescence was diffuse and indistinct, and endogenous spotty LC3 accumulation reduced in the platelets from ITP patients (Figure 1A).
In addition, LC3 protein expression was quantified by Western blotting. Results showed the conversion of LC3-I into LC3-II decreased in ITP patients, and the LC3-II/I ratio in ITP patients was significantly lower than in healthy controls (0.3380±0.0447 vs. 0.8440±0.0686, P<0.01; Figure 1B). These suggest platelet autophagy is suppressed in ITP patients.
Platelet autophagy was regulated by PI3K/AKT/mTOR pathway in ITP patients and healthy controls
Autophagy is regulated by several signaling pathways in nucleated cells (25). RAPA is the most commonly used trigger of autophagy, and mTOR as a target RAPA has been reported in the platelets from healthy controls. In our experiments, platelets from 20 ITP patients and 20 healthy controls were treated with RAPA in the presence or absence of 3-MA, a specific inhibitor of class III PtdIns3K. Upon RAPA treatment, immunofluorescence staining revealed the LC3-positive fluorescence increased significantly in the platelets from ITP patient. Furthermore, the effect of RAPA was inhibited by 3-MA (Figure 1A).
Similarly, Western blotting showed the LC3-II/I ratio in RAPA-treated ITP platelets was significantly higher than in un-treated ITP platelets (0.8960±0.1088 vs. 0.3380±0.0447, P<0.01) and was comparable to that of healthy controls (0.8960±0.1088 vs. 0.8440±0.06856, P=0.6906). The effect of RAPA was inhibited by 3-MA (0.8960±0.1088 vs. 0.3020±0.0550, P<0.01; Figure 1B). These suggest that platelet autophagy in ITP is regulated by the PI3K/AKT/mTOR signaling pathway and class III PtdIns3K plays a crucial role in the platelet autophagy of ITP patients.
Autophagic flux existed and was adjustable in platelet autophagy
p62 (also known as SQSTM1), a component of autophagosomes, is eliminated after formation of autolysosomes. p62 expression increases as autophagic flux is inhibited. Western blotting for p62 expression indicated a basal autophagic flux in ITP platelets. Treatment with NH4Cl or CQ, inhibitors of lysosomes, resulted in the accumulation of LC3-II in ITP platelets, consistent with RAPA treatment. Lysosomal inhibition resulted in marked accumulation of p62, contrary to RAPA treatment (Figure 1C).
Determination of ABO concentrations
ABO is a small-molecular compound which can efficiently enhance autophagy in nucleated cells. Whether ABO can also affect platelet autophagy is unknown. To evaluate the effects of ABO on platelet autophagy in ITP patients, platelets from 10 patients were treated with ABO at different concentrations (25 to 100 µmol/L). Western blotting revealed that ABO at different concentrations could increase the LC3-II/I ratio (0 µmol/L group, 0.1975±0.0452; 25 µmol/L group, 0.2975±0.0457; 50 µmol/L group, 0.9350±0.0829; 100 µmol/L group, 0.9025±0.0672), which reached a peak at 50 µmol/L (P<0.01 vs. 0 µmol/L), and there was no significant difference between 100 µmol/L group and 50 µmol/L group (P=0.2706 vs. 50 µmol/L; Figure 1D). Therefore, ABO can effectively induce platelet autophagy at 50 µmol/L. In addition, the effect of 50 µmol/L ABO was abolished by 3-MA (0.1750±0.0354, P<0.01; Figure 1D).
Platelet autophagy was induced by small-molecule compound ABO in ITP patients
When the platelets from 20 ITP patients were treated with 50 µmol/L ABO, a remarkable increase in LC3-positive fluorescence was observed (Figure 1A). Similarly, Western blotting indicated that the LC3-II/I ratio in ABO-treated ITP platelets was significantly higher than in untreated ITP platelets (0.8560±0.0970 vs. 0.3380±0.0447, P<0.01; Figure 1B). No significant difference was found between ABO-treated ITP platelets and RAPA-treated ITP platelets or platelets from healthy controls (0.8560±0.0970 vs. 0.8960±0.1088, P=0.1066; 0.8560±0.0970 vs. 0.8440±0.0686, P=0.4231; Figure 1B). Therefore, platelet autophagy can be induced by ABO in ITP patients, similar to the effect of RAPA.
Enhancing platelet autophagy alleviated ITP platelet destruction
To investigate whether enhancing autophagy suppressed ITP platelet destruction, the apoptosis and viability of platelets from 25 ITP patients and 25 healthy controls were further assessed. Platelets were treated with RAPA or ABO in the presence or absence of 3-MA. Results showed the apoptosis of ITP platelets was significantly higher than that of platelets from healthy controls (20.5837±2.5173 vs. 15.0232±1.7550, P<0.05; Figure 2). After treatment with RAPA, platelet apoptosis in ITP decreased significantly (14.7705±1.5651, P<0.01 vs. untreated ITP platelets), with no significant difference from platelets from healthy controls (P=0.6150). The effect of RAPA was abolished by 3-MA (20.9684±2.6443, P<0.01 vs. RAPA; Figure 2A,B).
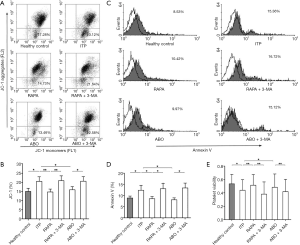
Similarly, after treatment with 50 µmol/L ABO, the apoptosis of ITP platelets decreased (15.9668±1.6660, P<0.05 vs. untreated ITP platelets), with no difference from that of platelets from healthy controls or RAPA-treated platelets (P=0.6988 vs. controls; P=0.0693 vs. RAPA). The effect of ABO was also inhibited by 3-MA (20.6711±2.5064, P<0.05 vs. ABO; Figure 2A,B).
After Annexin V staining, flow cytometry showed the platelet apoptosis in ITP was higher than in healthy controls (12.9862±3.6725 vs. 8.1269±1.6214, P<0.05; Figure 2C,D). After treatment with RAPA or ABO, the apoptosis in ITP platelets decreased significantly (RAPA: 7.9835±1.4117, P<0.05 vs. untreated ITP platelets; ABO: 7.5263±1.2823, P<0.05 vs. untreated ITP platelets), with no significant difference from that of platelets from healthy controls (RAPA: P=0.4912 vs. controls; ABO: P=0.3537 vs. controls). The effects of RAPA and ABO were inhibited by 3-MA (13.2469±3.8563, P<0.05 vs. RAPA; 13.7823±3.4937, P<0.05 vs. ABO; Figure 2C,D).
The platelet viability was lower in ITP patients than in healthy controls (0.4288±0.0404 vs. 0.5216±0.0394, P<0.05; Figure 2B). After RAPA treatment, the platelet viability improved significantly (0.5140±0.0393, P<0.01 vs. untreated ITP platelets), with no significant difference from that in healthy controls (P=0.6578 vs. controls). The effect of RAPA was inhibited by 3-MA (0.3848±0.0423, P<0.01 vs. RAPA; Figure 2B).
Consistent with the findings following RAPA treatment, after treatment with 50 µmol/L ABO, the platelet viability was increased significantly (0.4782±0.0447, P<0.05 vs. untreated ITP platelets), with no significant difference from RAPA-treated platelets and healthy control platelets (P=0.1255 vs. RAPA; P=0.4684 vs. controls). The effect of ABO was inhibited by 3-MA (0.4153±0.0424, P<0.01 vs. ABO; Figure 2B).
Discussion
Platelets are derived from bone marrow megakaryocyte cytoplasm and have a highly ordered cytoskeleton, lining systems, specialized secretory granules and receptors, and sensitive signaling pathways. Platelets contain a large amount of mRNAs from megakaryocytes, can also synthesize proteins and are involved in the regulation of a variety of physiological functions (26). However, little is known about the mechanism underlying the removal of misfolded or damaged proteins in platelets. Recent studies indicate that autophagy is observed in platelets and it is important for the hemostasis and thrombosis (16,27). In the present study, our results showed the platelet autophagy was significantly reduced in ITP patients as compared to healthy controls. This suggests that autophagy is suppressed in case of ITP, and the inhibition of platelet autophagy may be closely related to the platelet destruction in ITP.
In many autoimmune conditions, including systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis, there is evidence showing that their pathogenesis has involvement of abnormal autophagy (28-31). Autophagy can regulate the survival of autoreactive T cells and plays a more critical role in the peripheral T lymphocyte homeostasis than in the T cell development at earlier immature stages (32). As an autoimmune bleeding disorder, ITP is reported to be triggered by platelet antigens and subsequent pathogenic lymphocyte responses. Recently, our study showed aberrant expression of molecules in the autophagy pathway in the lymphocytes from ITP patients (33). In the present study, the relationship between abnormal autophagy and platelet destruction was investigated and the effects of platelet autophagy on the apoptosis and viability of platelets from ITP patients were further explored.
The autophagy of platelets from ITP patients was assessed by immunostaining assay and Western blotting, after treatment with RAPA in the presence or absence of 3-MA. Results showed RAPA treatment significantly enhanced the autophagy of ITP platelets, which could be inhibited by 3-MA. These results suggest the involvement of PI3K/Akt/mTOR pathway in the autophagy of ITP platelet.
Furthermore, the effect of ABO on the platelet autophagy was further evaluated in ITP patients. Cells were treated with ABO at different concentrations to determine the optical concentration of ABO. Results showed 50 µmol/L was optimal to stimulate autophagy of platelets from ITP patients. Similar to the findings after RAPA treatment, autophagy was induced by ABO in ITP platelets.
In our previous studies, ABO-induced autophagy was ascribed to the elevation of intracellular free Ca2+ in mTOR-independent and Annexin A7 (ANXA7)- dependent manners. ANXA7 is essential for the autophagy induction by modulating intracellular calcium concentration [(Ca2+)i] in human umbilical vein endothelial cells (24). ABO can also promote the interaction of ANXA7 with LC3 and grancalcin and regulate the phosphorylation of ANXA7 and relevant proteins. All these enhance autophagy of endothelial cells (34). Furthermore, ABO significantly reduces the secretion of inflammatory cytokines such as interleukin (IL)-6, IL-8, and phosphatidylcholine -specific phospholipase C (PC-PLC), promotes angiogenesis, and reduces p62 expression in the endothelial cells, maintaining the autophagy (35).
Although the relationship between apoptosis and autophagy is still controversial, increasing evidence supports their relationship, and regulating autophagy may alter the apoptosis of some types of cells in cancers and autoimmune diseases (25,36,37). To investigate whether regulating autophagy reduced platelet destruction, the viability and apoptosis of ITP platelets were evaluated after treatment with RAPA or ABO in the presence or absence of 3-MA. Results showed, after induction of autophagy with RAPA or ABO, the viability of ITP platelets improved and their apoptosis reduced. The effects of RAPA on the apoptosis and viability of platelets were inhibited by 3-MA.
Conclusions
In conclusion, platelet autophagy reduces in ITP patients. Platelet autophagy is regulated through the PI3K/AKT/mTOR signaling pathway and can also be regulated by the synthesized small-molecule compound ABO in ITP. Elevated platelet autophagy may prolong the life span of platelets from ITP patients by inhibiting platelet apoptosis and improving platelet viability. Our findings provide a novel mechanism underlying the pathogenesis of ITP and present a novel strategy for the treatment of ITP.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (No. 81500095, 91442204, 81770133, 81370623, 81770114 and 81470284), Shandong Provincial Natural Science Foundation, China (ZR2014HP066), and Tai Shan Scholar Foundation. The authors thank Mrs. Yuanyuan Zhu (Department of Hematology, Qilu Hospital, Shandong University) for technical assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Medical Ethical Committee of Qilu Hospital of Shandong University and the Second Hospital of Shandong University [IRB number: KYLL-2015(GJ)P-0016] and written informed consent was obtained from all patients.
References
- Li J, Sullivan JA, Ni H. Pathophysiology of immune thrombocytopenia. Curr Opin Hematol 2018;25:373-81. [PubMed]
- Liu XG, Bai XC, Chen FP, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol 2018;107:615-23. [Crossref] [PubMed]
- Catani L, Sollazzo D, Ricci F, et al. The CD47 pathway is deregulated in human immune thrombocytopenia. Exp Hematol 2011;39:486-94. [Crossref] [PubMed]
- Polverelli N, Catani L, Sollazzo D, et al. Platelet fluctuations during thrombopoietin-receptor agonist treatment: correlation with platelet apoptosis. Ann Hematol 2015;94:339-41. [Crossref] [PubMed]
- Min YN, Wang CY, Li XX, et al. Participation of B-cell-activating factor receptors in the pathogenesis of immune thrombocytopenia. J Thromb Haemost 2016;14:559-71. [Crossref] [PubMed]
- Zhou H, Qiu JH, Wang T, et al. Interleukin 27 inhibits cytotoxic T-lymphocyte-mediated platelet destruction in primary immune thrombocytopenia. Blood 2014;124:3316-9. [Crossref] [PubMed]
- Mariño G, Niso-Santano M, Baehrecke EH, et al. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 2014;15:81-94. [Crossref] [PubMed]
- Yang J, Yao S.. JNK-Bcl-2/Bcl-xL-Bax/Bak Pathway Mediates the Crosstalk between Matrine-Induced Autophagy and Apoptosis via Interplay with Beclin 1. Int J Mol Sci 2015;16:25744-58. [Crossref] [PubMed]
- Joubert PE, Werneke S, de la Calle C, et al. Chikungunya-induced cell death is limited by ER and oxidative stress-induced autophagy. Autophagy 2012;8:1261-3. [Crossref] [PubMed]
- Mo J, Zhang M, Marshall B, et al. Interplay of autophagy and apoptosis during murine cytomegalovirus infection of RPE cells. Mol Vis 2014;20:1161-73. [PubMed]
- Zhou A, Li S, Khan FA, et al. Autophagy postpones apoptotic cell death in PRRSV infection through Bad-Beclin1 interaction. Virulence 2016;7:98-109. [Crossref] [PubMed]
- Pott J, Kabat AM, Maloy KJ. Intestinal Epithelial Cell Autophagy Is Required to Protect against TNF-Induced Apoptosis during Chronic Colitis in Mice. Cell Host Microbe 2018;23:191-202.e4. [Crossref] [PubMed]
- Zhang Z, Zhang S, Wang Y, et al. Autophagy inhibits high glucose induced cardiac microvascular endothelial cells apoptosis by mTOR signal pathway. Apoptosis 2017;22:1510-23. [Crossref] [PubMed]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 2010;221:3-12. [Crossref] [PubMed]
- Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12:1-222.
- Feng W, Chang C, Luo D, et al. Dissection of autophagy in human platelets. Autophagy 2014;10:642-51. [Crossref] [PubMed]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009;43:67-93. [Crossref] [PubMed]
- Galluzzi L, Pietrocola F, Levine B, et al. Metabolic control of autophagy. Cell 2014;159:1263-76. [Crossref] [PubMed]
- Morgan MJ, Gamez G, Menke C, et al. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy 2014;10:1814-26. [Crossref] [PubMed]
- Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 2015;125:25-32. [Crossref] [PubMed]
- Mehrpour M, Esclatine A, Beau I, et al. Overview of macroautophagy regulation in mammalian cells. Cell Res 2010;20:748-62. [Crossref] [PubMed]
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A 1982;79:1889-92. [Crossref] [PubMed]
- Wang L, Dong Z, Huang B, et al. Distinct patterns of autophagy evoked by two benzoxazine derivatives in vascular endothelial cells. Autophagy 2010;6:1115-24. [Crossref] [PubMed]
- Li H, Liu N, Wang S, et al. Identification of a small molecule targeting annexin A7. Biochim Biophys Acta 2013;1833:2092-9. [Crossref] [PubMed]
- Zhang F, Zhao X, Shen H, et al. Molecular mechanisms of cell death in intervertebral disc degeneration Int J Mol Med 2016;37:1439-48. (Review). [Crossref] [PubMed]
- Frelinger AL 3rd, Grace RF, Gerrits AJ, et al. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood 2015;126:873-9. [Crossref] [PubMed]
- Ouseph MM, Huang Y, Banerjee M, et al. Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood 2015;126:1224-33. [Crossref] [PubMed]
- Caza TN, Talaber G, Perl A. Metabolic regulation of organelle homeostasis in lupus T cells. Clin Immunol 2012;144:200-13. [Crossref] [PubMed]
- Gros F, Arnold J, Page N, et al. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy 2012;8:1113-23. [Crossref] [PubMed]
- Lin NY, Stefanica A, Distler JH. Autophagy: a key pathway of TNF-induced inflammatory bone loss. Autophagy 2013;9:1253-5. [Crossref] [PubMed]
- Paunovic V, Petrovic IV, Milenkovic M, et al. Autophagy-independent increase of ATG5 expression in T cells of multiple sclerosis patients. J Neuroimmunol 2018;319:100-5. [Crossref] [PubMed]
- Jia T, Anandhan A, Massilamany C, et al. Association of Autophagy in the Cell Death Mediated by Dihydrotestosterone in Autoreactive T Cells Independent of Antigenic Stimulation. J Neuroimmune Pharmacol 2015;10:620-34. [Crossref] [PubMed]
- Shan NN, Dong LL, Zhang XM, et al. Targeting autophagy as a potential therapeutic approach for immune thrombocytopenia therapy. Crit Rev Oncol Hematol 2016;100:11-5. [Crossref] [PubMed]
- Ma H, Su L, Zhang S, et al. Inhibition of ANXA7 GTPase activity by a small molecule promotes HMBOX1 translation of vascular endothelial cells in vitro and in vivo. Int J Biochem Cell Biol 2016;79:33-40. [Crossref] [PubMed]
- Li H, Huang S, Wang S, et al. Targeting annexin A7 by a small molecule suppressed the activity of phosphatidylcholine-specific phospholipase C in vascular endothelial cells and inhibited atherosclerosis in apolipoprotein E(-)/(-)mice. Cell Death Dis 2013;4:e806. [Crossref] [PubMed]
- Al-Shenawy HA. Expression of Beclin-1, an autophagy-related marker, in chronic hepatitis and hepatocellular carcinoma and its relation with apoptotic markers. Apmis 2016;124:229-37. [Crossref] [PubMed]
- Chen HI, Tsai HP, Chen YT, et al. Autophagy and Apoptosis Play Opposing Roles in Overall Survival of Esophageal Squamous Cell Carcinoma. Pathol Oncol Res 2016;22:699-705. [Crossref] [PubMed]


