
The role of endobronchial ultrasound transbronchial needle aspiration for programmed death ligand 1 testing and next generation sequencing in advanced non-small cell lung cancer
Background and rationale for next-generation sequencing (NGS) and PD-L1 testing
NGS
NGS is a method which utilizes a single test to identify thousands of somatic or germline mutations from hundreds of genes (1). NGS is preferred over direct sequencing as it is more sensitive in specimens with low tumor cellularity (2), and may in fact be more cost-effective than single gene testing modalities. A recent report (that did not consider the cost of treatment) suggests that when compared to single gene testing modalities, NGS can save the Centers for Medicare & Medicaid Services (CMS) 1.4–2.1 million $ and commercial insurance providers >250,000 $ (3).
The best illustration of the impact NGS has on clinical practice is that driver mutations can be detected in 50–60% of patients with non-small cell lung cancer (NSCLC) (mainly non-squamous NSCLC and NSCLC NOS), of which approximately half can be treated with a targeted agent (2,4-6). Testing for EGFR mutations, ALK and ROS1 rearrangements can be considered (preferably by using broad molecular profiling) in patients with squamous cell carcinoma if they are never smokers, if small biopsy specimens were used for testing, or the tumor has mixed (adenosquamous) histology (7). The 2013 College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines endorsed by the American Society of Clinical Oncology recommended testing for EGFR mutations and ALK fusions in all patients with advanced-stage lung adenocarcinoma (8). Since then the number of lung cancer driver mutations that can be targeted by drugs has increased to include BRAF, ERBB2 (HER2), PIK3CA, AKT1, ROS1, RET, and MET amplifications (2). In 2015, NCCN guidelines expanded the recommended list of testing in this population to include ROS1, RET, BRAF, ERBB2, and MET (9). NCCN 2019 guidelines recommend testing using broad-based genomic sequencing (like NGS) to identify rare driver mutations and to assess clinical trial eligibility for other targeted treatments (7). The updated 2018 edition of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines now recommend that institutions either “(I) offer a comprehensive cancer panel that includes EGFR, ALK, ROS1, BRAF, MET, ERBB2 (HER2), KRAS and RET for all appropriate patients; or (II) offer targeted testing for the genes in the must-test category (EGFR, ALK, ROS1) for all appropriate patients and offer as a second test an expanded panel containing the second-category genes [BRAF, MET, ERBB2 (HER2), and RET] for patients who are suitable candidates for clinical trials (10)”.
Comprehensive genetic profiling with NGS is warranted not only at the time of initial diagnosis, but also when the mechanisms of resistance need to be evaluated at the time of disease progression. Re-biopsy at the time of disease progression has become standard of practice in patients treated with targeted therapy and is recommended by national guidelines (7). Complex resistance mechanisms can often only be identified with large gene panel testing, which is made feasible by NGS. However, a retrospective cohort study in a community setting in 5,688 patients with advanced NSCLC, broad-based genomic sequencing influenced treatment in only 4.5% (non-EGFR mutation or ALK rearrangement), and was not independently associated with improved survival (11). Most tertiary cancer centers, however, use large gene panels in their patients with advanced lung cancer for guiding further therapy (targeted agents, immunotherapy, chemotherapy or enrollment in clinical trials).
PD-L1 testing
Several immune checkpoint inhibitors are now available for use in patients with NSCLC (e.g., nivolumab, pembrolizumab, atezolizumab, durvalumab). Guidelines recommend testing for and quantification of expression of programmed death ligand 1 (PD-L1) expression on tumor cells at the time of diagnosis of advanced NSCLC (7,12). Testing should also be considered in patients who have disease progression with first-line chemotherapy. PD-L1 expression is determined by immunohistochemical (IHC) analysis.
Quantification is based upon a tumor proportion score (TPS) according to the percentage of viable tumor cells showing partial or complete membrane staining relative to all viable tumor cells present in the sample (13). From the 5,879 patients screened for eligibility in three major PD-L1 immune checkpoint inhibitor trials (KEYNOTE-001, KEYNOTE-010, KEYNOTE-024), 81% were evaluable for PD-L1 (33% with TPS <1%; 38% with TPS 1–49%; 28% with TPS ≥50%) (14,15). Response rates to pembrolizumab are greater in patients with tumors that have >50% PD-L1 expression (15,16). A recent study, however, showed that the benefit of immunotherapy was greater in patients with high tumor mutational burden, but was independent of histology and PD-L1 expression (>1% vs. <1%) (17). As of this writing, however, guidelines continue to recommend PD-L1 assessment by IHC analysis (9,12). Several antibodies and platforms exist for PD-L1 testing. Per the International Association for the Study of Lung Cancer (IASLC) statement on PD-L1 testing, “most antibodies, including those used exclusively in the laboratory-developed test setting, demonstrate comparable performance in well-controlled settings. Ultimately, the interpretation is limited by variable definitions of positivity for each companion or complementary diagnostic and by the lack of a clear gold-standard comparator, apart from the commercial kits themselves (18)”.
Role of EBUS for NGS and PD-L1 testing in advanced NSCLC
Guidelines for NSCLC recommend concomitant diagnosis, staging and acquisition of adequate material for genetic testing during the initial work-up of lung cancer (7,12). Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA), with its safety (19,20) and efficacy (sensitivity of up to 95% and specificity of ~100%) in confirming intrathoracic lymph node (LN) metastasis (21,22), is the recommended modality to stage the mediastinum in patients with NSCLC (23). In the following sections, we will discuss the role of EBUS-TBNA in obtaining adequate tissue for molecular analysis (i.e., NGS) and PD-L1 testing.
Evidence of EBUS-TBNA for NGS testing
Physicians involved in the care of patients with locally advanced or metastatic lung cancer must often rely on small cytological or histological biopsy samples to make a diagnosis and obtain adequate tissue for staging and ancillary studies. EBUS-TBNA samples were shown to be sufficient for identification of EGFR and ALK mutations in several studies. A pooled analysis of 28 studies (2,497 patients) reported sufficient sampling for EGFR in 94.48%, while an analysis of 12 studies (607 patients) reported sufficient sampling for ALK in 94.9% (24). EBUS-TBNA samples also appear adequate for ROS1 testing with a small study of 12 patients revealing adequate specimens in 83.3% patients (25).
As the list of recommended genetic alterations continues to expand (9), EBUS-TBNA samples continued to be assessed for their adequacy for increasingly comprehensive testing. A study in 54 patients (85 samples) demonstrated successful testing in 98% for a 50 gene panel and in 91.4% for a 1,213 gene panel, with no difference between the 22 and 25 G TBNA needles (26) (Figure 1). Of the successful tests, 85% were run on cytology smears, while the remaining 15% were on formalin-fixed, paraffin embedded (FFPE) cell-blocks. Another study of 115 patients showed that EBUS-TBNA obtained adequate specimens for NGS testing for large gene panels (341–469 genes) in 86.1% (99/115) of the cases (27). In this study, the source of DNA extraction was FFPE cell-blocks in 93 cases and cell-free DNA in needle rinse fluid in the 6 cases in which cell-block was inadequate. The authors’ success rate improved with time (76.3% for the first third and 92.3% for the last third), possibly due to improvement in sampling techniques or tissue processing.
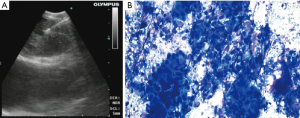
Methodology and technique
DNA requirements for NGS testing
Large-panel NGS testing (more than 200 genes) has been reported to require approximately 50 ng of DNA (2). The turn-around time and number of cells required, however, depend on the NGS platform. At our institution we use the Oncoscreen (50 genes) and OncoPlus (1,213 genes) panels—with the former requiring 1–10 ng of DNA (>1,000 cells) and a 7-day turn-around time; the latter requires 25–100 ng of DNA (>20,000 cells) and has a 14-day turn-around time.
Types of specimens for NGS testing
Compared to core biopsies, an advantage of using TBNA samples is that they are obtained via the least invasive method to sample a mediastinal, hilar or interlobar LN. Both FFPE cell-block samples (28,29) and cytology smears (30) have been shown to yield adequate and quality DNA samples for NGS testing. Formalin fixation and possibly the centrifugation required for cell block preparation in FFPE specimens may result in significant degradation of DNA (30,31). Alternatively, alcohol-based cytology fixatives (like Diff-Quik) may result in better preservation of high-quality nucleic acids and nuclear structure, providing a benefit in molecular testing (32). Besides, with smears, the ability to visualize the malignant cells on the slide and to enrich for malignant cells by selecting the appropriate slide or by microdissection, allows for an increased chance to detect mutations via NGS (30). At the University of Chicago, we routinely use cytology smears for NGS in lung cancer (Figure 1). There are several other successful reports of using smears for NGS in lung cancer from other tertiary cancer centers (26,33,34).
Number of needle passes for NGS testing
American College of Chest Physician guidelines recommend that “additional EBUS-TBNA samples beyond those needed to establish the diagnosis of NSCLC (≥3) be obtained for molecular analysis (20)”. There is no convincing data to guide physicians on the exact number of passes needed (24). In our study, we performed an average of 6 passes to result in enough material for IHC (for histologic subtyping of lung cancer) and for NGS (26).
Rapid on-site cytological evaluation
The use of rapid on-site evaluation (ROSE) helps optimize lung cancer genotyping by minimizing non-diagnostic samples and thereby preventing the need for a repeat procedure (35). In a study of 126 patients, genotyping was possible in 90% in the ROSE arm, compared with 80% in the arm without ROSE (36). Patients in the ROSE arm were more likely to have the bronchoscopy terminated after obtaining biopsies at a single site (58.9% vs. 44.1%). Subsequent studies on EBUS for NGS have used ROSE to increase success (26,27). This is because ROSE allows for slide cellularity and adequacy to be assessed at the time of the procedure, which cannot reliably be done with cell blocks. A recent perspective statement from the Pulmonary Pathology Society, recommends that when available, ROSE should be used with EBUS-TBNA in the diagnosis of lung cancer because it can minimize repeat procedures for additional desired testing such as molecular studies (35).
Evidence of EBUS TBNA for PD-L1 testing
Traditionally, testing for PD-L1 expression has relied on archived core tissue specimens. Therefore, there was an initial concern whether cytological samples, as obtained with EBUS-TBNA, would be reliable to test for PD-L1 expression. Heymann et al. studied PD-L1 testing adequacy from various samples and reported 90% (36 of 40) adequacy with cytology specimens, a rate comparable to those obtained from small biopsies or surgical specimens (37). Another study of 97 NSCLC samples found that EBUS-TBNA collected significantly larger number of tumor cells compared to transbronchial biopsy (median 1,149 vs. 435) and was associated with lower crush artifacts (38). Subsequent studies have shown that the EBUS-TBNA procedure can offer sufficient samples for PD-L1 IHC testing in 85–90% of specimens (39,40).
The perceived concern with PD-L1 testing on small biopsies is its imperfect correlation with testing performed on larger specimens reported in some studies. In fact, one study of 160 patients demonstrated a 48% discordance rate between surgical specimens and small biopsies [mainly transbronchial biopsies (69%) and CT-guided lung biopsies (24%)] (41). However, subsequent studies using EBUS-TBNA have demonstrated lower discordance rates. In a small study, Sakakibara et al. showed that EBUS-TBNA samples had good concordance with the corresponding primary tumor (r=0.75; n=6) as well as with LN metastasis (r=0.93; n=5) (38). In a study of 61 patients who underwent EBUS-TBNA followed by surgical resection, when compared to the surgically resected specimens, EBUS-TBNA had a sensitivity of 72% to identify PD-L1 expression of ≥1% and only 47% to identify PD-L1 expression of ≥50% (42). This is concerning as 20–30% of NSCLCs have PD-L1 expression >50% and the intensity of expression may predict better outcomes with certain immunotherapy agents (15,42). Concordance rates for PD-L1 ≥1% and ≥50% were 87% and 82%, respectively (42). Another study of 161 samples demonstrated an overall concordance rate of 75.2% between primary and metastatic tumor sites, suggesting good correlation (43). When the tumors were dichotomized using PD-L1 cut-offs of 1% and 50%, the concordance rate increased by greater than 10%. A plausible explanation for discordance may be intra-tumoral heterogeneity, which has been described in 10–19% of cases (44,45). While not supported by all studies (42), there is some evidence to suggest that PD-L1 expression is altered by neoadjuvant therapy (46,47). There are also observer dependent difficulties in quantifying PD-L1 expression. The conditions of a cell-block differ from those of a resected specimen. In cell-blocks, cells are fragmented and scattered within the clot, with normal tissue fragments and blood being embedded within the tumor. Another challenge in quantifying PD-L1 expression has been the varying proportion of tumor and stromal cells in each patient’s tumor (39). In our opinion, in cases in which EBUS-TBNA shows pure tumor on ROSE, the cell block may have higher tumor cellularity, in which case PD-L1 staining may be more reliable (Figure 2).
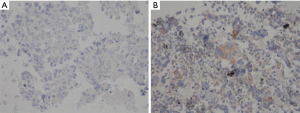
Methodology and technique
Assays: several assays exists for PD-L1 testing (Dako 22C3-pembrolizumab, Dako 28-2-nivolumab, Ventana SP142-atezolizumab), with evidence suggesting good correlation between tests (13,48,49).
Specimens: while traditionally tested on cell blocks or core biopsy samples, recent evidence suggests good concordance between these and cytology smears. Noll et al. analyzed samples which had at least 100 tumor cells for adequacy of PD-L1 testing (37 smears and 38 cell blocks) (50). Smears and cell blocks had a 97% and 82%, respectively, concordance rate with paired core needle biopsies. However, PD-L1 testing on cytology smears is not yet standard of practice (18).
Technique: small specimen sampling can lead to misclassification of a patient’s PD-L1 status due to intra-tumoral heterogeneity in PD-L1 expression (44,45). Targeting different areas of the LN through fanning technique could improve adequacy of the sampled specimen and potentially overcome discordance due to intra-tumoral heterogeneity (51). It is unclear as to how many passes are required to obtain sufficient samples for accurately determining PD-L1 expression. False negative cases are often a consequence of low cellularity samples; with one study showing that ~80% false negative samples had <1,000 tumor cells (42). This problem can be potentially alleviated by performing more passes and with the use of ROSE for real-time feedback. Biswas et al. used a median of 6 passes (range 5–8) from a particular site to obtain a cell-block for both NGS and PD-L1 testing using a 22 G needle (39). There is no evidence to suggest that EBUS needle gauge influences accuracy of PD-L1 testing. Small gauge needles (22 and 25 G) have also been shown to yield adequate samples for PD-L1 testing (39,40). There are no studies on the use of the 19 G needle or transbronchial LN forceps biopsy for PD-L1 testing. However, reportedly, 19 G needles could provide more cellular material compared with 21 G needles (52). Whether this difference results in improved specimen adequacy for PD-L1 testing with EBUS-TBNA, remains to be studied.
Factors to consider prior to biopsy at the time of disease progression
NGS allows for genomic profiling of tumors that enables physicians to personalize therapy by choosing the most appropriate molecularly targeted drug (53). However, given the varied mechanisms of resistance to targeted therapies, including epithelial to mesenchymal transformations and transformation into small cell lung cancers, it is essential to reassess these tumors with repeat biopsies at the time of disease progression (54). Repeat biopsy can confirm disease progression and evaluate for secondary mutations which might offer prognostic value and help guide referral towards clinical trials. Studies showed that the majority of patients with progression on first generation tyrosine kinase inhibitors (i.e., erlotinib) develop resistance due to a mutation or other genetic alteration that is targetable (54). In fact, guidelines recommend such molecular testing at the time of disease progression (7). In one study, NGS resulted in a change in management in 24.4% patients who were tested at the initial diagnosis (n=41) and in 84.6% of patients who were tested at the time of progression of disease (n=13) (26).
While considering repeat biopsies in patient who are suspected to have progression on immune checkpoint inhibitors, the treating team should be aware of progression patterns.
Pseudo-progression (PP), refers to transient increase in size of a tumor or metastatic sites (confirmed on biopsy as inflammatory cellular infiltrates or necrosis) or the development of new lesions, with subsequent regression (55-57). This occurs in 2–6% of tumors (depending upon the criteria used to define this condition) and can occur with any of the immune checkpoint inhibitors. The mean time to progression is 74 days while the mean time to response is 169 days (58,59). As PP can mimic true progression, clinician judgement is required to determine the need for a biopsy. Follow-up imaging in ≥4–8 weeks is recommended in most cases to distinguish PP from true progression (56). Modified RECIST (RECIST version 1.1) criteria classify tumor responses as complete response, partial response, stable disease, and progressive disease. These descriptors could not capture the group of patients who had PP. Hence, the iRECIST, the latest iteration of classification system assessing tumor response, further stratifies tumors as either unconfirmed or confirmed progressive disease (iUPD or iCPD) (56). The irRC and iRECIST criteria are more accurate in classifying radiologic PP as non-progression (57).
Hyper-progressive disease (HPD), on the other hand, is a paradoxical rapid progression of the tumor with worsening of clinical status, that negatively impacts survival (60,61). This is defined as ≥2-fold increase in tumor growth rate at 8 weeks (based on the primary lesion). The incidence rate is ~10% and is the same with both PD-1 (i.e., nivolumab and pembrolizumab) and PD-L1 (i.e., atezolizumab and durvalumab) antibodies (59-61). The mechanism of HPD is unclear, but is thought to be related to: (I) oncogenic signaling activation (affecting alternative signaling networks to enhance tumor growth); or (II) the upregulation of alternative immune check points; or (III) the modulation of other protumor immune subsets; or (IV) due to tumor escape as a consequence of activation of tumor lymphocytes-related local inflammation, angiogenesis, tissue remodeling or metabolism modification (61). We believe that re-biopsy in patient who progresses on immunotherapy is relevant as it may help confirm PP or HPD, two entities with obvious distinct management strategies.
Summary
Driver mutations and PD-L1 tumor expression are detected in 50–60% and ~80% of patients with advanced NSCLC, respectively. Guidelines recommend testing for driver mutations using broad-based genomic sequencing, and testing for and quantification of PD-L1 expression on tumor cells. These should be done at the time of initial diagnosis as well at disease progression. EBUS-TBNA can provide adequate sample for NGS testing, with both FFPE and cytology smears yielding high-quality DNA samples for large-panel NGS testing. FFPE cell-blocks and cytology smears obtained via EBUS-TBNA have been shown to be sufficient for PD-L1 testing and quantification, but the latter has not yet been sufficiently validated. These samples have good concordance with the primary tumor and distant metastases. For both NGS and PD-L1 testing, needle size does not seem to influence the yield, while ROSE may help increase the yield by enabling intra-procedural cellularity and adequacy determination. While the number of passes needed is not clear, 4-6 passes seems to be the minimum at this point. At the time of disease progression in patients on targeted agents, repeat biopsies should be considered to determine the mechanism of resistance and to assess eligibility for other targeted therapy, immunotherapy, chemotherapy or enrollment in clinical trials. In patients on immunotherapy who have tumor growth within the first 3 months of initiation of immune checkpoint inhibitors, a re-biopsy may be warranted to distinguish PP and hyper- progression prior to deciding on change of therapy.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Murgu has acted as a paid educational consultant for Olympus, Cook Inc., Pinnacle Biologics and Boston Scientific. Dr. Hogarth has received honoraria for preceptorships, lectures, and consulting from Boston Scientific. He has also received unrestricted education grants from Boston Scientific. He has received honorarium from Biodesix and Veracyte for lectures and consulting. He has received unrestricted education grants from Biodesix. He has been part of contracted research studies for Veracyte. The other author has no conflicts of interest to declare.
References
- Blumenthal GM, Mansfield E, Pazdur R. Next-Generation Sequencing in Oncology in the Era of Precision Medicine. JAMA Oncol 2016;2:13-4. [Crossref] [PubMed]
- Drilon A, Wang L, Arcila ME, et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin Cancer Res 2015;21:3631-9. [Crossref] [PubMed]
- Pennell NA, Mutebi A, Zhou Z, et al. Economic impact of next generation sequencing vs sequential single- gene testing modalities to detect genomic alterations in metastatic non-small cell lung cancer using a decision analytic model. J Clin Oncol 2018;36:abstr 9031.
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Lim SM, Kim EY, Kim HR, et al. Genomic profiling of lung adenocarcinoma patients reveals therapeutic targets and confers clinical benefit when standard molecular testing is negative. Oncotarget 2016;7:24172-8. [Crossref] [PubMed]
- Kaderbhai CG, Boidot R, Beltjens F, et al. Use of dedicated gene panel sequencing using next generation sequencing to improve the personalized care of lung cancer. Oncotarget 2016;7:24860-70. [Crossref] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-small cell lung cancer. Version 3.2019-January 18, 2019.). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol 2014;32:3673-9. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018;20:129-59. [Crossref] [PubMed]
- Presley CJ, Tang D, Soulos PR, et al. Association of Broad-Based Genomic Sequencing With Survival Among Patients With Advanced Non-Small Cell Lung Cancer in the Community Oncology Setting. JAMA 2018;320:469-77. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Neuman T, London M, Kania-almog J, et al. A Harmonization Study for the Use of 22C3 PD-L1 Immunohistochemical Staining on Ventana's Platform. J Thorac Oncol 2016;11:1863-8. [Crossref] [PubMed]
- Aggarwal C, Abreu DR, Felip E, et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann Oncol 2016;27:1060P. [Crossref]
- Reck M, Rodríguez-abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- IASLC Atlas of PD-L1 Immunohistochemistry Testing in Lung Cancer. Available online: https://www.iaslc.org/sites/default/files/wysiwyg-assets/iaslc_pd-l1_atlas_mar2018_lo-res.pdf
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care 2015;60:1040-50. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012;186:255-60. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Labarca G, Folch E, Jantz M, et al. Adequacy of Samples Obtained by Endobronchial Ultrasound with Transbronchial Needle Aspiration for Molecular Analysis in Patients with Non-Small Cell Lung Cancer. Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2018;15:1205-16. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Pires Y, et al. Molecular Testing of EGFR, EGFR Resistance Mutation, ALK and ROS1 Achieved by EBUS-TBNA in Chile. Arch Bronconeumol 2017;53:172-4. [PubMed]
- Stoy SP, Segal JP, Mueller J, et al. Feasibility of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Cytology Specimens for Next Generation Sequencing in Non-small-cell Lung Cancer. Clin Lung Cancer 2018;19:230-8.e2. [Crossref] [PubMed]
- Turner SR, Buonocore D, Desmeules P, et al. Feasibility of endobronchial ultrasound transbronchial needle aspiration for massively parallel next-generation sequencing in thoracic cancer patients. Lung Cancer 2018;119:85-90. [Crossref] [PubMed]
- Qiu T, Guo H, Zhao H, et al. Next-generation sequencing for molecular diagnosis of lung adenocarcinoma specimens obtained by fine needle aspiration cytology. Sci Rep 2015;5:11317. [Crossref] [PubMed]
- Young G, Wang K, He J, et al. Clinical next-generation sequencing successfully applied to fine-needle aspirations of pulmonary and pancreatic neoplasms. Cancer Cytopathol 2013;121:688-94. [Crossref] [PubMed]
- Treece AL, Montgomery ND, Patel NM, et al. FNA smears as a potential source of DNA for targeted next-generation sequencing of lung adenocarcinomas. Cancer Cytopathol 2016;124:406-14. [Crossref] [PubMed]
- Vincek V, Nassiri M, Nadji M, et al. A tissue fixative that protects macromolecules (DNA, RNA, and protein) and histomorphology in clinical samples. Lab Invest 2003;83:1427-35. [Crossref] [PubMed]
- Fischer AH, Cibas ES, Howell LP, et al. Role of cytology in the management of non-small-cell lung cancer. J Clin Oncol 2011;29:3331-2. [Crossref] [PubMed]
- Malapelle U, Mayo-de-las-casas C, Molina-vila MA, et al. Consistency and reproducibility of next-generation sequencing and other multigene mutational assays: A worldwide ring trial study on quantitative cytological molecular reference specimens. Cancer Cytopathol 2017;125:615-26. [Crossref] [PubMed]
- Baum JE, Zhang P, Hoda RS, et al. Accuracy of next-generation sequencing for the identification of clinically relevant variants in cytology smears in lung adenocarcinoma. Cancer Cytopathol 2017;125:398-406. [Crossref] [PubMed]
- Jain D, Allen TC, Aisner DL, et al. Rapid On-Site Evaluation of Endobronchial Ultrasound-Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2018;142:253-62. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017;125:896-907. [Crossref] [PubMed]
- Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a Promising Method for the Evaluation of Tumor PD-L1 Expression in Lung Cancer. Clin Lung Cancer 2017;18:527-34.e1. [Crossref] [PubMed]
- Biswas A, Leon ME, Drew P, et al. Clinical performance of endobronchial ultrasound-guided transbronchial needle aspiration for assessing programmed death ligand-1 expression in nonsmall cell lung cancer. Diagn Cytopathol 2018;46:378-83. [Crossref] [PubMed]
- Stoy SP, Rosen L, Mueller J, et al. Programmed death-ligand 1 testing of lung cancer cytology specimens obtained with bronchoscopy. Cancer Cytopathol 2018;126:122-8. [Crossref] [PubMed]
- Ilie M, Long-mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016;27:147-53. [Crossref] [PubMed]
- Sakata KK, Midthun DE, Mullon JJ, et al. Comparison of Programmed Death Ligand-1 Immunohistochemical Staining Between Endobronchial Ultrasound Transbronchial Needle Aspiration and Resected Lung Cancer Specimens. Chest 2018;154:827-37. [Crossref] [PubMed]
- Kim S, Koh J, Kwon D, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer 2017;75:141-9. [Crossref] [PubMed]
- Casadevall D, Clavé S, Taus Á, et al. Heterogeneity of Tumor and Immune Cell PD-L1 Expression and Lymphocyte Counts in Surgical NSCLC Samples. Clin Lung Cancer 2017;18:682-91.e5. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Fujimoto D, Uehara K, Sato Y, et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep 2017;7:11373. [Crossref] [PubMed]
- Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep 2016;6:20090. [Crossref] [PubMed]
- Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:3585-91. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Noll B, Wang WL, Gong Y, et al. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol 2018;126:342-52. [Crossref] [PubMed]
- Murgu SD. Diagnosing and staging lung cancer involving the mediastinum. Chest 2015;147:1401-12. [Crossref] [PubMed]
- Pickering EM, Holden VK, Heath JE, et al. Tissue Acquisition During EBUS-TBNA: Comparison of Cell Blocks Obtained From a 19G Versus 21G Needle. J Bronchology Interv Pulmonol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Tsao AS, Scagliotti GV, Bunn PA, et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol 2016;11:613-38. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol 2015;33:3541-3. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Reckamp KL. Real-World Pseudoprogression: an Uncommon Phenomenon. J Thorac Oncol 2018;13:880-2. [Crossref] [PubMed]
- Kurra V, Sullivan RJ, Gainor JF, et al. Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J Clin Oncol 2016;34:abstr 6580.
- Kurman JS, Murgu S. Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J Thorac Dis 2018;10:1124-8. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]


