
Effect of Bushen Huoxue decoction on inhibiting osteogenic differentiation of vascular smooth cells by regulating OPG/RANK/RANKL system in vascular calcification
Introduction
Cardiovascular disease (CVD) is the predominant cause of mortality in patients with chronic kidney disease (CKD) and may be related to excess vascular calcification (VC). Among CKD patients, the presence and extent of VC independently predicts the risk of CVD and the related mortality (1). VC is a highly regulated process with the hallmark of phenotypic changes of vascular smooth muscle cells (VSMCs) to an osteogenic phenotype, together with calcified extracellular matrix surrounding the VSMCs. This calcification course has features similar to calcification that occurs in bone and is usually induced by hyperphosphatemia in the uremia environment (2). Thus, we usually make use of the hyperphosphate medium to intervene the VSMCs and establish a VC model in vitro. Osteoprotegerin (OPG), receptor activator of nuclear factor-kappa B (RANK) and receptor activator of nuclear factor-kappa B ligand (RANKL) are all members of the tumor necrosis factor receptor family. As many in the field know, the OPG/RANK/RANKL system plays an important role in bone metabolism. Recently, studies have cultivated new interest in its potential role in VC in CKD (3,4). Evidence is accumulating that OPG may prevents RANKL interaction and subsequent stimulation with its receptor, RANK, thereby suppressing the promotion of osteogenic differentiation of VSMCs and therefore protect against VC (5). These proteins may play a vital role in regulating the development of VC coincident with disordered bone metabolism in CKD. Despite the many measures that have been proposed to alleviate VC, the treatment of VC-related disease such as disordered mineral homeostasis and bone metabolism continues to perplex and researchers in its complexity.
Chinese medicine (CM) has been widely used for thousands of years and a series of studies have suggested that kidney-nourishing methods could bring multiple benefits to people with CKD due to their effectiveness in improving kidney function and alleviating symptoms of uremia (6-8). In particular, Bushen Huoxue Decoction (BSHXD), a Chinese prescription of Professor Ning Zhang, has been found to be potentially capable of alleviating the symptoms of renal osteodystrophy and hemodialysis patients (9). In addition, it has shown to be able to regulate the bone metabolism and mineral homeostasis in CKD rats (10). Furthermore, in our previous study, BSHXD up-regulated the expression of OPG mRNA and down-regulated the expression of RANKL mRNA in osteoblast cells of mice in vitro (11). Most importantly, our previous study also demonstrated that BSHXD alleviated the extent of aorta calcification in CKD Sprague-Dawley (SD) rats in vivo induced by adenine gavage and high phosphorus dietary (12). Thus, we conducted the current experiment to investigate the effects of BSHXD on inhibiting osteogenic differentiation of VSMCs and explore the related mechanism by regulating the OPG/RANK/RANKL system in VC.
Methods
Medicated serum preparation
Sixty male SD rats weighing 180–200 g were randomly divided into two groups. Each group contained 30 rats, and they were used for preparation of serum containing tested drugs. Control group rats were given distilled water for 2 days, twice a day, and the other groups were given BSHXD (3 g/mL). Blood was obtained aseptically from the abdominal aortas of the SD rats 2 h after the final administration, and serum was then obtained via centrifugation of the blood at 3,000 r/min for 10 minutes. Following inactivation in 56 °C water for 30 minutes and filtrated with a 0.22 µm cellulose acetate membrane, the serum was then bottled and stored at −80 °C for use.
VSMCs culturing conditions
VSMCs from the rats were purchased from the ATCC cell library in Shanghai. The VSMCs were cultured in Dulbecco’s modified eagle medium (DMEM) containing 20% fetal bovine serum (FBS, Gibco, USA) and were incubated at 37 °C in 5% CO2. Cells were plated (8×104 cells/plate) in 6 plates. For the experiments, cells were divided into five groups as follows: the normal group (treated with medium plus 20% normal rat serum), the model group (treated with 2.4 mmol/L NaH2PO4 and medium plus 20% normal rat serum), the BSHXD-H group (treated with 2.4 mmol/L NaH2PO4 and medium plus 20% BSHXD serum), the BSHXD-M group (treated with 2.4 mmol/L NaH2PO4 and medium plus 10% BSHXD serum and 10% normal rat serum), and the BSHXD-L group (treated with 2.4 mmol/L NaH2PO4 and medium plus 5% BSHXD serum and 15% normal rat serum). To confirm consistency in different groups, the normal rat serum to replenish the concentration to 20% in both the middle group and low groups. The mediums were changed every 2 days.
Drug
BSHXD consists of the following ingredients: Astragali radix, prepared Radix rehmanniae, Psoralea corylifolia, Herba epimedii, Salvia miltiorrhiza, Angelica sinensis, Rheum officinale, Rhizoma cibotii, Dipsacus asper, Oyster shell. All of the drugs were purchased from the pharmacy of Wang Jing hospital, China Academy of Chinese Medical Science. We also analyzed the chemical constituents of BSHXD by high-performance liquid chromatography (HPLC) (Figure S1).
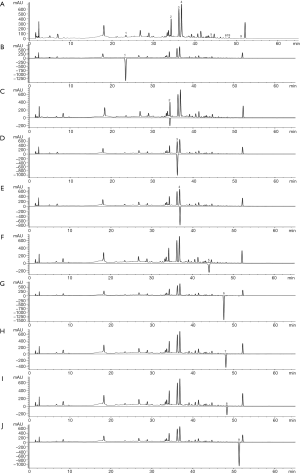
Alizarin red S staining-based calcium deposition analysis
On the 10th day after culturing, the medium was removed and rinsed the cells twice with PBS, and then fixed with 95% alcohol, and washed with distilled water. The calcium deposition area of each group was examined by using an alizarin red S staining (Genmed Scientifics Inc., USA).
Determination of VSMCs calcium content
The calcium levels were measured in each group on the same day of treatments, as well as on the 2nd, 4th, 6th, 8th, and 10th days after the addition of the treatments. The calcium content was measured using the Calcium Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, decalcified the cells with 0.6 N HCl for 24 hours. After that, washed the cells for 3 times with PBS and solubilized them with 0.1 N NaOH/0.1% sodium dodecyl sulfate (SDS). The protein content was measured with the CBB Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The absorbance of the calcium and the protein were determined at 610 and 590 nm respectively using a plate reader. The calcium content of the cell layer was normalized to total cellular protein.
Measurement of alkaline phosphatase (ALP) activity
The ALP activity was measured on the same day of treatments, as well as on the 2nd, 4th, 6th, 8th, and 10th days after the addition of the treatments. Cells were lysed by 0.1% Triton X-100 overnight at 4 °C. The ALP activity was determined by p-nitrophenyl phosphate (pNPP) using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The absorbance was determined at 520 nm using a plate reader. The ALP activity of the cell layer was normalized to total cellular protein.
Western blot analysis of α-smooth muscle actin (α-SMA) and ALP
α-SMA and ALP protein VSMCs were measured on the 3rd, 6th, and 10th day. VSMCs were washed with an ice-cold PBS and then lysed with RIPA. After a brief sonication using a sonicator, cell debris was separated by centrifugation at 15,000 rpm for 10 min at 4 °C. Supernatant protein concentrations were measured by a BCA kit. Separated the proteins on 10% SDS-PAGE gel and then transferred them to PVDF membranes. After being blocked with 5% non-fat milk for 1 h, the membranes were incubated with special antibodies directed against α-SMA and ALP (1:10,000, Abcam) or β-actin overnight at 4 °C. The catalog number of the special antibodies of α-SMA and ALP are ab7817 and ab108337 respectively. After 4 washes with TBST, the membranes were incubated with secondary antibody (the catalog number: cw0103) (1:10,000) for 1 h. After extensive washing with TBST, blots were observed with an enhanced chemiluminescence kit and imaged using a ChemiDoc XRS system. Densitometry of the immunoblot data analysis was performed using Quantity One.
Quantitative real-time polymerase chain reaction analysis of OPG and RANKL
Cells lysed in Trizol were sonicated using a sonicator, and extracted total RNA with chloroform, precipitated them with isopropyl alcohol, and resuspended them in diethyl pyrocarbonate (DEPC) treated water. cDNA was generated from total RNA using a FastQuant RT kit. OPG and RANKL mRNA expression were tested by a qSTAR SYBR Master Mix-Low Rox Kit using an ABI 7500 Fast real-time PCR System. PCR conditions consisted of 95 °C for 15 minutes followed by 40 cycles at 95 °C for 10 seconds, 62 °C for 30 seconds, and increased 5°C from 75 °C until it reached 95 °C. The primers were 5'-TCAGAAAGGAAATGCAACAC-3' and 5'-CGGTATAATCTTGGTAGGCAC-3' for detection of OPG, 5'-TTCAGAATTGCCCGACCAGTTTTT-3' and 5'-CCCAGACATTTGCACACCTCAC-3' for detection of RANKL, and 5'-TGAGGTGACCGCATCTTCTTG-3' and 5'-TGGTAACCAGGCGTCCGATA-3' for detection of GAPDH, which was used as a reference.
Statistical analysis
The results are presented as means ± standard errors of the mean (SEMs). Statistical significance was analyzed via one-way analysis of variance (ANOVA), followed by Tukey’s test for multiple comparisons. Notably, data of TER was analyzed by ANOVA with replicated measures. Findings of P<0.05 were considered statistically significant.
Results
BSHXD attenuates the calcium deposition of the VSMCs
Calcium deposition of the VSMCs were examined by alizarin red S staining (Figure 1). At 10 days after alizarin red S staining, the model group wells were fully covered by mineralized deposits, suggesting the osteogenic differentiation of VSMCs was caused by high levels of phosphate. Although there were no obvious changes in the BSHXD-L group, the mineralized deposits decreased distinctly both in the BSHXD-M group and the BSHXD-H group.
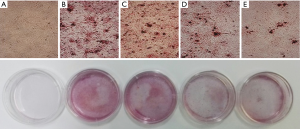
BSHXD alleviates calcium content of the calcified VSMCs
We detected the calcium content using the Calcium Kit. As shown in Figure 2, compared with the normal group, the calcium content increased with the reaction time in the model group, especially in the end stage of the experiment. While the calcium content significantly decreased in the BSHXD-M group and the BSHXD-H group, both of them showed significant difference (Figure 2).
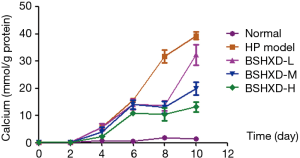
BSHXD reduces ALP activity of the calcified VSMCs
We detected the ALP activity using a commercial assay kit. As shown in Figure 3, the cells in the model group presented higher activity compared with them in normal group, while the treatment group demonstrated lower activity. Additionally, the BSHXD-H group showed the most dramatic difference (Figure 3).
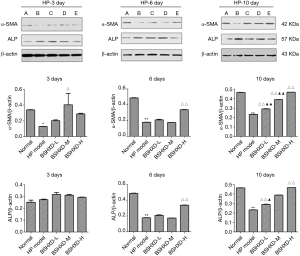
BSHXD increased expression of α-SMA protein and decreased expression of ALP protein
We detected expression of α-SMA and ALP protein, the maker proteins of the VSMCs and the osteoblast respectively, using Western blot. As shown in Figure 4, the levels of α-SMA protein were greatly decreased and the ALP protein increased in the model group, compared with those in normal group. Meanwhile, the expression of α-SMA and ALP protein in the treatment groups of BSHXD showed significantly higher and lower on the 10th day, respectively, compared with the model group (Figure 4).
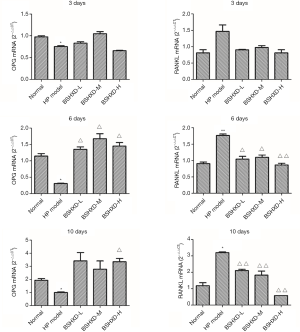
BSHXD up-regulates the expression of OPG mRNA and down-regulates the expression of RANKL mRNA
We detected the expression of OPG mRNA and RANKL mRNA using RT-PCR. As shown in Figure 5, the expression of OPG mRNA in the model group demonstrated a significant decrease and RANKL mRNA increase, compared with the normal group. However, the expression of OPG mRNA in BSHXD-H-treated group was higher than that in the model group, and this effect was imprecise in the BSHXD-L and BSHXD-M-treated group. Meanwhile, the expression of RANKL mRNA in all BSHXD-treated groups were lower than those in the model group (Figure 5).
Discussion
CKD patients develop accelerated VC and the calcification rapidly progresses in dialysis patients (13). Importantly, VC strongly contributes to the high risk of cardiovascular morbidity and mortality in CKD patients.
VC was in the past considered to be an imminent, passive, and degenerative process involving advanced vascular lesions; however, recent research has revealed numerous similarities with actively controlled processes occurring in the bone tissue (14). This complex and active process involves numbers of mechanisms responsible for calcium depositions on arterial walls. They lead to an augment in arterial stiffness and in pulse wave velocity, which in turn contributes to the high risk of CVD morbidity and mortality. The key mechanism of VC has been suggested to be VSMCs differentiation to osteogenic cells with the presence of VSMCs calcification simulating factors in CKD, such as inflammation factors and higher concentration of circulating nucleators, such as phosphate (15,16). High concentration of phosphate due to the declining kidney function and dysregulated mineral metabolism induces VSMCs to transform to osteogenic-phenotype cells, which has been found coincidence with the loss of VSMC marker proteins (α-SMA, SM22α, etc.) characteristics, and the gain of osteoblast cell marker proteins (such as ALP, etc.) (17,18). Serum P is now recognized as a main risk factor for cardiovascular events in CKD2–4 (19). Also, relatively minor elevations in serum P have been related with increased risk of cardiovascular mortality in CKD patients (20). In our study, we found that the VSMCs calcification was successfully induced on the 10th day via being cultured in a high concentration of phosphate (2.4 mM), showing obvious calcium deposition of the VSMCs in the model group examined by alizarin red S staining, which was consistent with the report of Jono et al. (18). At the same time, the expression of the a-SMA, the VSMC marker protein, was down-regulated, while the expression of the ALP, the osteoblast cell marker proteins, was up-regulated.
It is well known that OPG is a soluble decoy receptor for RANKL and is involved in osteoclast development and calcification (21). OPG acts as a molecule that prevents RANKL interaction and subsequent stimulation with its receptor, RANK, thereby suppressing the promotion of the calcification process in a bone morphogenetic protein (BMP, i.e., BMP-2 and BMP-4)-dependent manner (21,22). Additionally, studies have cultivated new interest in its potential role in VC in CKD, and it has become increasingly accepted that OPG inhibits VC (23). As Zhang et al. reported (24), OPG-Fc could attenuate calcification of MSCs induced by osteogenic differentiation media. Schoppet et al. (25) confirmed that OPG overexpression in vitro inhibited VSMC calcification and other studies have suggested there was a reduction in the calcificated VSMCs infected with OPG overexpression lentiviral vector (17). Just like what OPG do in osteoclast development, OPG acts as an inhibitor that prevents RANKL interaction and subsequent stimulation with its receptor, RANK, thereby suppressing the promotion of osteogenic differentiation of VSMCs (5). Hence, the OPG/RANKL pathway plays a vital role in cell differentiation and maturation, as well as in VC (26). Current researches on the mechanism of VC mainly focus on calcium and phosphorus metabolism and the balance of calcification including promoting and inhibitory factors of calcification. More underlying regulatory mechanisms should be explored.
CM is widely used in CKD and an evidence-based application of CM is gradually increasing. Several, large clinical studies have indicated the beneficial effects of Chinese herbal medicine in CKD patients (6-8). However, CM interventions for VC in CKD are lacking. The aims of this study were to evaluate the effects of BSHXD and its underlying molecular mechanisms on inhibiting VC via the OPG/RANKL pathway.
In our previous study, BSHXD was found to be able to regulate bone metabolism and mineral homeostasis, and importantly, to reduce the extent of aorta calcification in CKD rats (10). In this study, we demonstrated that BSHXD could regulate the differentiation of VSMCs into osteoblasts via the OPG/RANKL pathway in a dose-dependent manner.
BSHXD reduced the mineralized deposits distinctly at 10 days after alizarin red S staining, especially in the BSHXD-M group and the BSHXD-H group. At the same time, the expression of the a-SMA, the VSMC marker protein, was up-regulated, while the expression of the ALP, the osteoblast cell marker proteins, was down-regulated in the BSHXD-H group at 10 days. Both the calcium content and the ALP activity were reduced in the BSHXD-M and the BSHXD-H group with the reaction time, but the effect was not obvious in the BSHXD-L group. In conclusion, BSHXD’s effect in preventing the phenotypic changes of VSMCs to an osteogenic phenotype appears promising. This study also indicated that BSHXD up-regulated the expression of OPG mRNA at 10 days and coincidently down-regulated the expression of RANKL mRNA, which may be the related mechanism of the effect of BSHXD on inhibiting the VC in CKD. The effect was not fully manifested on the 3rd day, at the beginning of the study, but the effect became apparent later on. The BSHXD-H group demonstrated a significant and lasting effect, compared with the BSHXD-L and BSHXD-M group.
In summary, BSHXD has a beneficial effect on inhibiting osteogenic differentiation of VSMCs induced by high levels of phosphate. The underlying mechanism appears to be related to the modulation of expressions of OPG mRNA and RANKL mRNA in the VSMCs, thereby preventing the phenotypic changes of VSMCs to an osteogenic phenotype.
Acknowledgements
Funding: This work was supported by the National Nature Science Foundation of China (No. 81273747) and the Innovation Talent Training Foundation for Doctoral Students of China Academy of Chinese Medicine Sciences (No. CX201506).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Animal Ethics Committee of Wang Jing Hospital, China Academy of Chinese Medical Sciences, Beijing (No. 20130507).
References
- Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001;38:938-42. [Crossref] [PubMed]
- Kendrick J, Chonchol M. The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis 2011;58:826-34. [Crossref] [PubMed]
- Bucay N, Sarosi I, Dunstan CR, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998;12:1260-8. [Crossref] [PubMed]
- Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 2004;95:1046-57. [Crossref] [PubMed]
- Ndip A, Williams A, Jude EB, et al. The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes 2011;60:2187-96. [Crossref] [PubMed]
- Wang YJ, He LQ, Sun W, et al. Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol 2012;139:757-64. [Crossref] [PubMed]
- Dong F, Cheng J, Lin S, et al. The clinical research on serum cystatin-C alteration on stage II chronic kidney disease with gubenquduyishen decoction treatment. J Ethnopharmacol 2010;131:581-4. [Crossref] [PubMed]
- Li S, Rao XR, Dai XW, et al. Beneficial effects of Fu-Zheng-Qu-Zhuo oral liquid combined with standard integrated therapy in patients with chronic kidney disease (stage 3-4): A randomized placebo-controlled clinical trial. Medicine (Baltimore) 2017;96:e7448. [Crossref] [PubMed]
- Zhang N, Liu SW, Ren K, et al. Influences of therapy of reinforcing kidney and activating blood on parathormone and alkaline phosphatase of patient with renal osteopathy. Journal of Beijing University of Traditional Chinese Medicine 2005;(4):1-3.
- Zhang N, Zhang YZ, Qi EJ, et al. Experimental study on the improvement of bone metabolism abnormality in rats with renal failure by invigorating kidney and activating blood circulation. Chinese Medical Journal 2000.68-70.
- Lin Y, Zhang N, Liu SW, et al. Experimental research for effect of Bushenhuoxue recipe on HPTH(1-34) intervened osteoblasts and long-term clinical observation for treatment of renal osteodystrophy. Chinese Journal of Clinicians 2012.3065-8. (Electronic Edition).
- Liu SY, Zhang N, Meng XF, et al. Effect of Bushen Huoxue Decotion on inhibiting vascular calcification in chronic kidney disease rats by regulating BMP-2/Runx2/Osterix signal pathway. Chin J Integr Tradit Western Med 2016;39:2013-9.
- Bellasi A, Kooienga L, Block GA, et al. How long is the warranty period for nil or low coronary artery calcium in patients new to hemodialysis? J Nephrol 2009;22:255-62. [PubMed]
- Jeziorska M, McCollum C, Wooley DE, et al. Observations on bone formation and remodeling in advanced atherosclerotic lesions of human carotid arteries. Virchows Arch 1998;433:559-65. [Crossref] [PubMed]
- Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial 2007;20:103-9. [Crossref] [PubMed]
- Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int 2009;75:890-7. [Crossref] [PubMed]
- Son BK, Akishita M, Iijima K, et al. Mechanism of pi-induced vascular calcification. J Atheroscler Thromb 2008;15:63-8. [Crossref] [PubMed]
- Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000;87:E10-7. [Crossref] [PubMed]
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009;20:381-7. [Crossref] [PubMed]
- Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005;16:520-8. [Crossref] [PubMed]
- Sun H, Li Q, Zhang Y, et al. Regulation of OPG and RANKL expressed by human dental follicle cells in osteoclastogenesis. Cell Tissue Res 2015;362:399-405. [Crossref] [PubMed]
- Steinmetz M, Skowasch D, Wernert N, et al. Differential profile of the OPG/RANKL/RANK-system in degenerative aortic native and bioprosthetic valves. J Heart Valve Dis 2008;17:187-93. [PubMed]
- Davaine JM, Quillard T, Chatelais M, et al. Bone Like Arterial Calcification in Femoral Atherosclerotic Lesions: Prevalence and Role of Osteoprotegerin and Pericytes. Eur J Vasc Endovasc Surg 2016;51:259-67. [Crossref] [PubMed]
- Zhang Q, Chen S, Shi J, et al. Coupled OPG-Fc on Decellularized Aortic Valves by EDC/NHS Attenuates Rat MSCs Calcification In Vitro. ASAIO J 2019;65:197-204. [Crossref] [PubMed]
- Schoppet M, Kavurma MM, Hofbauer LC, et al. Crystallizing nanoparticles derived from vascular smooth muscle cells contain the calcification inhibitor osteoprotegerin. Biochem Biophys Res Commun 2011;407:103-7. [Crossref] [PubMed]
- Abedin M, Omland T, Ueland T, et al. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am J Cardiol 2007;99:513-8. [Crossref] [PubMed]


