
Is an immune checkpoint inhibitor really a hopeless therapeutic choice for EGFR-mutant non-small cell lung cancer (NSCLC) patients?
The treatment strategy of lung cancer with chemotherapy is changing rapidly owing to developments in the characterization of non-small cell lung cancer (NSCLC) genetic profiles and identification of hallmark immunological characteristics (1). Indeed, this represents a time of unprecedented changes occurring within a short period. These advances in knowledge and technologies have led to the development of several targeted therapies and immune checkpoint inhibitors directed against tumor molecules, which also serve as therapeutic biomarkers. Currently available clinical routine biomarkers guiding the treatment of patients with NSCLC include epidermal growth factor receptor (EGFR) mutations, the T790M EGFR resistance mutation, anaplastic lymphoma kinase (ALK) fusion gene status, ROS1 fusion gene status, EGFR expression, and programmed death-ligand 1 (PD-L1) expression (2). However, implementation of these molecular biomarkers in clinical practice remains challenging, and the application of genomic and immunological biomarkers in routine clinical practice has raised several concerns that have yet to be resolved by the large clinical studies conducted to date. These questions include: What is the overlap of a PD-L1 tumor proportion score (TPS) of at least 50% with the conccurent targetable driver oncogenic mutations? How should one select the appropriate 1st line chemotherapy on the basis of the biomarker profile? In this commentary, we focus on the treatment strategy for EGFR-mutant NSCLC patients with high PD-L1 expression.
In this regard, the specific association between EGFR mutation and PD-L1 expression in NSCLC remains unclear. Some studies using surgical samples of chemotherapy naïve NSCLC patients have demonstrated lower PD-L1 TPS in EGFR-mutant than in EGFR wild-type tumors (3,4), whereas others have demonstrated the opposite result (5,6). Thus, this issue remains controversial. Notably, several studies demonstrated the possible poorer efficacy of anti-PD-1 antibodies for treating EGFR-mutant NSCLC patients (7-9). A pooled analysis including data of the major clinical studies conducted to date confirmed that immune checkpoint inhibitors do not enhance the overall survival (OS) of EGFR-mutant NSCLC patients compared with that of patients taking docetaxel (HR =1.05, P<0.81; treatment-mutation interaction P=0.03) (10). Another pooled analysis covering five clinical trials (Checkmate 017 and 057, Keynote 010, OAK, POPLAR) verified that immune checkpoint inhibitors prolonged OS in the EGFR wild-type subgroup but not in the EGFR-mutant subgroup (11). There has been much speculation regarding this apparent limited benefit of immune checkpoint inhibitors in EGFR-mutant NSCLC patients. A recent report reported that a lack of T cell infiltration, tumor immunogenicity and a significantly decreased mutation burden cause an inferior response to PD-1 inhibitors in EGFR-mutated NSCLC patients (12).
A phase II trial (NCT0287994) was conducted to test the efficacy of pembrolizumab in tyrosine kinase inhibitor (TKI)-naive EGFR-mutant advanced NSCLC patients with high PD-L1 expression (13). However, enrolment was ceased owing to the lack of efficacy after 11 of the 25 planned patients received the treatment. Although the number of patients in this study was limited due to premature closure for futility, the data do not support a significant benefit of administering pembrolizumab prior to EGFR-TKI treatment. Thus, first-line pembrolizumab treatment in TKI-naive advanced NSCLC patients with EGFR mutation is not an appropriate choice. Intriguingly, this trial also showed that EGFR-TKI was still efficacious after pembrolizumab failure, with no effect of the preceding immune checkpoint inhibitors on the treatment. From these findings, the National Comprehensive Cancer Network (NCCN) clinical practice guidelines of NSCLC (version 3, 2018) indicate that immune checkpoint inhibitors are less effective in EGFR-mutant NSCLC patients regardless of PD-L1 expression. Therefore, the NCCN guidelines do not recommend immune checkpoint inhibitors for the treatment of EGFR-mutant NSCLC patients.
Thus, we are left with a key question as to whether an immune checkpoint inhibitor is really a hopeless therapeutic choice for EGFR-mutant lung cancer. Recently, we reported a patient with high PD-L1-expressing (TPS 90%) NSCLC harboring EGFR-mutation (Ex.19 deletion) who did not respond to erlotinib as the first-line therapy but dramatically responded to pembrolizumab as the second-line therapy, which was attributed to intratumor heterogeneity of the PD-L1-expressing and EGFR-mutant clones (14). Multiple immunofluorescent staining analysis could successfully discriminate between EGFR-mutant and PD-L1 highly expressing clones. The immunofluorescent image and digital-droplet PCR analysis revealed sparse representation of the EGFR-mutant clones in the tumor. Uenami et al. (15) reported two EGFR-mutant NSCLC patients with high PD-L1-expressing tumors who both showed a good response to pembrolizumab after EGFR-TKI. Moreover, Taniguchi et al. (16) reported three NSCLC patients with a rare, minor EGFR mutation (G719X) who were also effectively treated with pembrolizumab. These reports indicate that immune checkpoint inhibitors could in fact be an effective treatment for EGFR-mutant NSCLC patients.
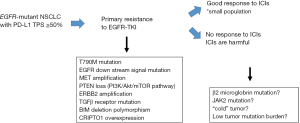
From the perspective of EGFR-TKI efficacy, high PD-L1 expression is associated with primary resistance to EGFR-TKI in NSCLC patients harbouring EGFR-mutation (17,18). Patients with a greater than 50% PD-L1-positive tumor cells had a significant risk of acquiring primary resistance to EGFR-TKIs than patients with PD-L1 TPS <50% (odds ratio, 16.47; 95% confidence interval, 2.10–129.16; P=0.008) (17). Several possible mechanisms regarding primary resistance to EGFR-TKI in EGFR-mutant NSCLC patients have been proposed to date, including BIM deletion polymorphism, co-existence of MET amplification, PTEN loss, ERBB2 amplification, and KRAS mutation (19). However, the precise mechanism by which PD-L1 expression is associated with primary resistance to EGFR-TKI remains unclear. The mechanism how tumor cells express PD-L1 on their surface are regulated by two major pathways (20): an “extrinsic” mechanism in which an antitumor cellular immune response driven by interferon-gamma from tumor infiltrative lymphocytes, which in turn induces PD-L1 expression on tumor cells; and an “intrinsic” mechanism, in which constitutive oncogenic signaling leads to PD-L1 expression. The expression of PD-L1 was controlled by an EGFR- and JAK2, STAT1-dependent manner in head and neck cancer cells. Aberrant EGFR signaling promotes intrinsic PD-L1 expression in EGFR-mutant NSCLC cells, which may not play a role in influencing the working point of immune checkpoint inhibitors.
The potential of the combination of EGFR-TKI and an immune checkpoint inhibitor has not yet been fully evaluated in preclinical models. However, several early phase clinical trials have evaluated this strategy. The TATTON trial is a multiphase Ib trial, in which osimertinib is combined with durvalumab (21). Both EGFR-TKI pretreated and naive patients were included in the study and the objective response rate (ORR) was 67% and 21% in T790M positive and negative cases, respectively. The ORR was also 67% in EGFR-TKI naïve patients, which are similar to those obtained with osimertinib alone. The adverse effect rate of interstitial pneumonia was similar in both groups. Consequently, further enrolment of the TATTON trial has been stopped (21). Furthermore, a phase III trial evaluating the combination of durvalumab with osimertinib compared to osimertinib alone in patients with EGFR T790M positive NSCLC patients has also been suspended. Unexpectedly, these studies demonstrated a high incidence of adverse events with the combination therapy. However, preliminary results from other early studies have shown promising efficacy and acceptable toxicity. Specifically, in the phase I study of nivolumab (CheckMate 012), 21 EGFR-mutant NSCLC patients were treated with the combination of nivolumab and erlotinib associated with an acceptable toxicity profile (22). The ORR was 19%, with 3 of the 20 EGFR-TKI-pretreated patients and the one EGFR-TKI naïve patient achieving a partial response. The study reveals that the combination therapy of erlotinib and nivolumab had an acceptable safety profile. Furthermore, the treatment is well efficacy in EGFR-mutant NSCLC patients resistant to previous EGFR-TKI therapy. Preliminary results are now also available on the safety and efficacy of the combination of erlotinib plus atezolizumab from the other processing study in advanced NSCLC patients (23). The study consists of a safety evaluation stage independently of EGFR mutation status followed by an expansion phase in EGFR-mutant NSCLC patients. They reported durable clinical responses and a manageable safety profile. Unfortunately, no clear synergistic effect of the combination has been observed and grade >3 adverse events were more common. However, these results are still preliminary and long-term safety and efficacy data are awaited in addition.
EGFR mutation tests provide a binary result: presence or absence of EGFR mutation. Thus, the traditional lung cancer treatment strategy was far simpler than the present situation demands. In particular, a treatment strategy with immune checkpoint inhibitors must be reconsidered in the face of targetable driver mutations in a cross-sectoral manner. As reviewed herein, recent studies have revealed some meaningful findings in this regard. First, pembrolizumab is not recommended as the first-line treatment for EGFR-TKI-naive, PD-L1-high expression (TPS >50%), EGFR-mutant NSCLC cases (13). Second, immune checkpoint inhibitors can be a promising treatment option for some PD-L1-high expression, EGFR-mutant NSCLC cases (14-16). Third, EGFR-TKI as the first-line treatment can be unsuccessful for EGFR-TKI-naive, PD-L1-high expression, EGFR-mutant NSCLC cases owing to the strong possibility of primary resistance to EGFR-TKI (17,18). Given this complexity, it is essential to identify the key factor for achieving the success of immune checkpoint inhibitors for PD-L1-high expression, EGFR-mutant NSCLC patients. Toward this end, further evaluation focusing on biomarkers is warranted in EGFR-mutant NSCLC patients to identify those who might derive the greatest benefit from this treatment (Figure 1). Moreover, given that multiple co-occurring oncogenic mutations are present in the vast majority of advanced-stage EGFR-mutant NSCLC patients (24), more informed and genomically empowered molecular diagnosis is critical for determining the most appropriate treatment strategy, including tumor microenvironment analysis. These efforts are essential, as patients with advanced NSCLC cannot afford more than one treatment failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bernicker EH, Miller RA, Cagle PT. Biomarkers for selection of therapy for adenocarcinoma of the lung. J Oncol Pract 2017;13:221-7. [Crossref] [PubMed]
- Gandara DR, Riess JW, Kelly K, et al. Evolution and increasing complexity of the therapeutic landscape in advanced Non-Small-cell Lung Cancer. Clin Lung Cancer 2017;18:1-4. [Crossref] [PubMed]
- Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol 2016;11:1879-90. [Crossref] [PubMed]
- Hata A, Katakami N, Nanjo S, et al. Programmed death-ligand 1 expression according to epidermal growth factor receptor mutation status in pretreated non-small cell lung cancer. Oncotarget 2017;8:113807-16. [Crossref] [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. [Crossref] [PubMed]
- D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95-102. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-smallcell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomized controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Dong ZY, Wu SP, Liao RQ, et al. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumour Biol 2016;37:4251-61. [Crossref] [PubMed]
- Lisberg A, Cummings A, Goldman JW, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol 2018;13:1138-45. [Crossref] [PubMed]
- Kunimasa K, Nakamura H, Sakai K, et al. Heterogeneity of EGFR-mutant clones and PD-L1 highly expressing clones affects treatment efficacy of EGFR-TKI and PD-1 inhibitor. Ann Oncol 2018;29:2145-7. [Crossref] [PubMed]
- Uenami T, Ishijima M, Kanazu M, et al. Two cases of response to pembrolizumab in epidermal growth factor receptor mutated lung adenocarcinoma patients with programmed death-ligand 1 overexpression. Ann Transl Med 2018;6:444. [Crossref] [PubMed]
- Taniguchi Y, Tamiya A, Ishii S, et al. Effect of pembrolizumab on patients harboring uncommon epidermal growth factor receptor mutations. Ann Oncol 2018;29:1331-3. [Crossref] [PubMed]
- Hsu KH, Huang YH, Tseng JS, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naïve advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer 2019;127:37-43. [Crossref] [PubMed]
- Su S, Dong ZY, Xie Z, et al. Strong programmed death ligand 1 expression predicts poor response and De Novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol 2018;13:1668-75. [Crossref] [PubMed]
- Zhong J, Li L, Wang Z, et al. Potential resistance mechanisms revealed by targeted sequencing from lung adenocarcinoma patients with primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). J Thorac Oncol 2017;12:1766-78. [Crossref] [PubMed]
- Concha-Benavente F, Srivastava RM, Trivedi S, et al. Identification of the cell-Intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res 2016;76:1031-43. [Crossref] [PubMed]
- Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref]
- Gettinger S, Chow LQ, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. Int J Radiat Oncol Biol Phys 2014;90:S34-5.
- Rudin C, Cervantes A, Dowlati A, et al. P3.02c-046 Safety, Clinical activity and biomarker results from a phase Ib study of erlotinib plus atezolizumab in advanced NSCLC. J Thorac Oncol 2017;12:S1302-3. [Crossref]
- Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 2017;49:1693-704. [Crossref] [PubMed]


