Adjuvant therapy in renal cell carcinoma—is pharmacogenomics assessment another element to select our patients?
Adjuvant therapy in resected renal cell carcinoma (RCC) is a discussed and complex issue due to conflicting results obtained in several clinical trials exploring different agents in this setting (1-4).
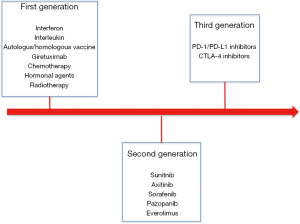
Of note, looking to postoperative systemic treatments we need to describe three different types of drugs tested in different times and thus three different waves of randomized clinical trials (Figure 1).
First generation consisted on studies evaluating the impact of “old’’ immunotherapy represented mainly by interferon, autologous vaccine and interleukin (1). Only one study evaluating an autologous tumour cell vaccine showed an improvement in disease free survival (DFS); however, due to the high cost of production and to some issues related to study design and course (unblended assignment, imbalance in patient baseline characteristics and high number of drop-outs after randomization) this approach has not been included in clinical practice (1,2).
Second generation of studies is represented by target agents and mainly by agents able to target angiogenesis. Sunitinib, sorafenib, pazopanib and axitinib have been tested in four randomized clinical trials (4-11). Of these trials only S-TRAC study comparing sunitinib to placebo showed an improvement in DFS for patients receiving sunitinib. The improved benefit was observed in first interim analysis and then confirmed after a longer follow up in all risk categories according to University of California Los Angeles (UCLA) integrated staging system (6,7). However, it is important to specify that no studies have demonstrated that adjuvant tyrosine kinase inhibitors (TKIs) are associated to improved overall survival (OS). Even the already described S-TRAC has not reached median overall survival in both treatment arms.
There are several issues, which could partially explain the different results observed in terms of DFS among these clinical trials. First of all, the different compounds adopted. Indeed, even if each of these compounds (sunitinib, sorafenib, pazopanib and axitinib) have shown to improve clinical outcomes in metastatic setting it important observe that each of them interacts and inhibits a specific spectrum of TK receptors and thus specific and different pathways. Of course, the main activity of these drugs is directed against the Vascular Endothelial Factor Receptors family (VEGFRs), however we do not have to forget that other pathways could be inhibited by these agents and the role of these on the early phases of metastases development is far to be understood. Other clinical issues that should be considered are correlated to the selection of patients among clinical trials (2). Indeed, each study has adopted a different system of tumour staging and this could have affected recruitment of patients resulting on possible different population on study (with different risk of tumour relapse). Modality of drug administration is another important issue as ASSURE trial reported a not negligible percentage of patients who received a dose reduction during treatment period. If we consider PROTECT trial, primary and secondary analysis on patients receiving pazopanib at 600 and 800 mg daily respectively showed an improved DFS benefit in 800 mg population (8,12). Thus, the modality and dosage of drug administration could be an important factor that should be considered even if it results in worst toxicity profiles.
What have we learned from these second-generation studies?
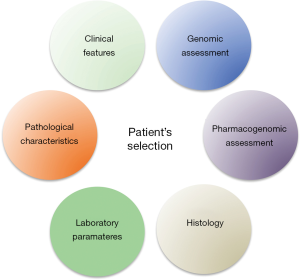
The strongest message emerging from the results of these studies is probably that selection of patients is one of the most important aspects to consider in adjuvant treatment. We have several scores, which could be adopted to reach this aim, however these scores adopted clinical, pathological and laboratory parameters to estimate the risk of recurrence and prognosis of this patients and maybe this may not be enough anymore.
In last years an increasing deal of studies has investigated which are the major genomic alterations occurring in renal cell carcinoma (13-16). Results provided by the assessment seem to introduce us in an extremely complex world in which RCC emerges as a heterogeneous disease associated to different genomic mutations which drive different clinical outcomes.
Are the current staging systems sufficient enough to estimate our patient’s prognosis?
Maybe the inclusion of parameters able to integrate molecular and genomic information to the available criteria could be a winning strategy. In 2015, Rini et al. described and validated a 16-panel gene profile to estimate clinical outcomes of patients with resected tumours (17). However, it is possible that future challenges about genomic characterization of tumours will also focus on the evaluation of sensitivity to a specific treatment approach other than prognosis and risk of tumour recurrence.
In addition, to a better understanding of tumour genomic assessment other issues regarding interaction between treatment-tumour and host could be of particular interest.
In this line, George et al. published in November 2018 results of an exploratory pharmacogenomics analysis carried out on 286 patients enrolled in S-TRAC studies (18). In this analysis, authors evaluated specific single-nucleotide polymorphisms (SNPs) of 11 selected genes and correlation between SNPs and outcome after adjuvant treatment. They observed that longer DFS could be observed in patients receiving placebo and expressing specific SNP including: VEGFR1 rs9554320 C/C (HR =0.44; 95% CI, 0.21–0.91; P=0.023), VEGFR2 rs2071559 T/T (HR =0.46; 95% CI, 0.23–0.90; P=0.020), and eNOS rs2070744 T/T (HR =0.53; 95% CI, 0.30–0.94; P=0.028). Of note, shorter DFS was observed for VEGFR1 rs9582036 C/A versus C/C with sunitinib, placebo, and combined therapies (P<0.05), and A/A versus C/C with sunitinib (P=0.022). VEGFR1 rs9554320 A/C versus A/A was associated with shorter DFS in the placebo (P=0.038) and combined (P=0.006) groups. Of note, previous study exploring association between SNP and patient’s clinical outcomes in metastatic setting failed to show an association between SNPs (VEGFA rs699947, VEGFA rs1570360, VEGFR3 rs448012, VEGFR3 rs307821, and VEGFR3 rs307826) and progression free survival (PFS), OS, objective response rate and time to tumor progression. Moreover, the small number of patients in which this analysis has been carried out does not allow us to reach final conclusions about the role of SNPs. The planning of further prospective and validation studies is necessary to confirm these observations. However, this study proposes to consider another factor strictly related to host-treatment-tumor interaction to differentiate and select patients and so increase our precision during enrollment of patients in adjuvant and maybe also advanced setting trial.
How can we do better?
One of the most important future challenges will be probably the inclusion of other factors and assessment during initial evaluation of our patients (Figure 2). We know that genomic assessment of the disease is strictly related with specific outcomes of our patients including risk of recurrence. However, more efforts should be spent on the research of correlation between genomic assessment and response to treatment in the optic of “precision medicine”. The inclusion of genomic assessment of the disease could provide important information to clinicians and thus improve our ability to select patients to enroll in adjuvant trials. Furthermore, the study of Daniel et al. with other previous similar evaluation open a new interesting scenario in which also consideration about specific polymorphisms related to key genes could strongly modulate clinical outcomes during treatment (18). This issue should be considered and further evaluated in perspective studies.
Are we ready for third generation?
The advent of immune-checkpoint inhibitors has drastically improved clinical outcomes of our patients in metastatic setting in both previously treated and previously untreated patients (19). As known these agents have shown to increase survival of our patients and are currently one of the most effective treatment options in metastatic RCC. However, few it is known about their role in metastatic setting and several adjuvant trials are still on going. Again, selection of patients has been performed with the same criteria adopted in second-generation studies. Nonetheless, retrospective genomic assessments as well as other pharmacogenomics analysis are important opportunities that should be performed to confirm our previous observation and maybe develop integrated and validated criteria able to provide important information (in terms of prognosis and response to treatment) to clinicians.
Think of a fourth generation?
Increasing evidences seems to confer to combination strategies between TKIs and immune-checkpoint inhibitors a very promising role in terms of response rate and PFS, OS improvement in metastatic setting (20-22). However, considering the results observed by TKIs alone in second-generation studies (results from EVEREST studies evaluating everolimus as adjuvant treatment are still awaiting) and the ongoing phase III studies with immune-checkpoint inhibitors alone it is difficult to imagine that combination treatment will be tested soon in adjuvant setting. Nonetheless, combination treatment remains a promising approach and maybe it could be also an important opportunity to test new and integrated criteria for patient selection.
In conclusion, to date we still do not know if adjuvant treatment results in an effective OS improvement. DFS improvement could reflect a specific genomic assessment and maybe could be also associated to specific SNP of key target genes. Future challenges should be focused on the evaluation of criteria able to include genomic assessment of the disease, confirmation and validation of these reported pharmacogenomics observations and to the investigation of the role of immune-checkpoint inhibitors in this setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Massari F, Bria E, Maines F, et al. Adjuvant treatment for resected renal cell carcinoma: are all strategies equally negative? Potential implications for trial design with targeted agents. Clin Genitourin Cancer 2013;11:471-6. [Crossref] [PubMed]
- Massari F, Di Nunno V, Ciccarese C, et al. Adjuvant therapy in renal cell carcinoma. Cancer Treat Rev 2017;60:152-7. [Crossref] [PubMed]
- Massari F, Di Nunno V, Ardizzoni A. Re: Robert J. Motzer, Alain Ravaud, Jean-Jacques Patard, et al. Adjuvant Sunitinib for High-risk Renal Cell Carcinoma After Nephrectomy: Subgroup Analyses and Updated Overall Survival Results. Eur Urol. In press. http://dx.doi.org/. Eur Urol 2017. [Epub ahead of print]. [Crossref]
- Sun M, Marconi L, Eisen T, et al. Adjuvant Vascular Endothelial Growth Factor-targeted Therapy in Renal Cell Carcinoma: A Systematic Review and Pooled Analysis. Eur Urol 2018;74:611-20. [Crossref] [PubMed]
- Massari F, Di Nunno V, Mollica V, et al. Adjuvant Tyrosine Kinase Inhibitors (Tki) In Renal Cell Carcinoma: A Meta-Analysis Of Available Clinical Trials. Clin Genitourinary Cancer 2019. [Epub ahead of print]. [Crossref]
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375:2246-54. [Crossref] [PubMed]
- Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant Sunitinib for High-risk Renal Cell Carcinoma After Nephrectomy: Subgroup Analyses and Updated Overall Survival Results. Eur Urol 2018;73:62-8. [Crossref] [PubMed]
- Motzer RJ, Haas NB, Donskov F, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol 2017;35:3916-23. [Crossref] [PubMed]
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, place-bo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008-16. [Crossref] [PubMed]
- Haas NB, Manola J, Dutcher JP, et al. Adjuvant Treatment for High-Risk Clear Cell Re-nal Cancer: Updated Results of a High-Risk Subset of the ASSURE Randomized Trial. JAMA Oncol 2017;3:1249-52. [Crossref] [PubMed]
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol 2018;29:2371-8. [Crossref] [PubMed]
- Stewart GD, Leibovich BC, Negrier S, et al. Adjuvant Pazopanib Does Not PROTECT Against Recurrence of High-Risk, Initially Localized Renal Cell Cancer but Does Provide Novel Insights. J Clin Oncol 2017;35:3895-7. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 2016;374:135-45. [Crossref] [PubMed]
- Ricketts CJ, De Cubas AA, Fan H, et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep 2018;23:3698. [Crossref] [PubMed]
- Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014;26:319-30. [Crossref] [PubMed]
- Rini B, Goddard A, Knezevic D, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol 2015;16:676-85. [Crossref] [PubMed]
- George DJ, Martini JF, Staehler M, et al. Phase III Trial of Adjuvant Sunitinib in Patients with High-Risk Renal Cell Carcinoma: Exploratory Pharmacogenomic Analysis. Clin Cancer Res 2019;25:1165-73. [Crossref] [PubMed]
- Santoni M, Massari F, Di Nunno V, et al. Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context 2018;7:212528. [Crossref] [PubMed]
- Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol 2018;19:405-15. [Crossref] [PubMed]
- Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol 2018;19:451-60. [Crossref] [PubMed]
- Di Nunno V, Santoni M, Massari F. Re: Michael B. Atkins, Elizabeth R. Plimack, Igor Puzanov, et al. Axitinib in Combination with Pembrolizumab in Patients with Advanced Renal Cell Cancer: A Non-randomised, Open-label, Dose-finding, and Dose-expansion Phase 1b Trial. Lancet Oncol 2018;19:405-15. Eur Urol 2018;74:e50. [Crossref] [PubMed]