Outcomes of transcatheter aortic valve replacement in bicuspid aortic valve stenosis
Introduction
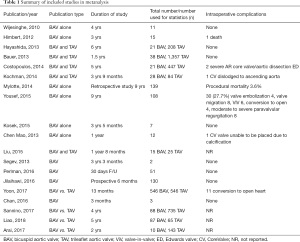
Transcatheter aortic valve replacement (TAVR) has become an acceptable therapy for the management of severe symptomatic aortic stenosis in patients deemed inoperable or high risk for conventional surgical aortic valve replacement (SAVR) (1-3). Due to abnormal valve geometry and asymmetric annulus with a presumed risk for residual paravalvular leak (PVL), prosthesis malposition and malfunction, patient with bicuspid aortic valve (BAV) have been excluded in many TAVR trials (1,2) resulting in very limited data with regards to its safety and efficacy. Consequently, despite the known benefits of TAVR, current guidelines have excluded patients with BAV from their recommendations (3-5). Given that 20% of stenotic aortic valves in patients greater than 80 years of age are in fact bicuspid in nature (6), this presents a unique therapeutic challenge in a large patient population with no viable alternative once they have been deemed inoperable or extremely high risk for open heart surgery. The consensus that BAV is not readily amenable to TAVR is not based on randomized controlled studies but rather on anecdotal evidence and limited case series showing lower device success rates (7-11). Studies have suggested the lower rate of device success may be due to BAV being more calcified (12) and having asymmetric annulus (2), two factors that increase the difficulty of the TAVR procedure. However, there have been numerous case series and cohort studies (Table 1) which show that TAVR in carefully selected patients with BAV is feasible, safe and may have a similar outcome to TAVR in tri-leaflet aortic valve (TAV) stenosis. Recently, large multinational BAV registries (13-15) and meta-analyses (16,17) on post-procedural outcomes in BAV patients have been impressive. To our knowledge, our study is the largest and most current systematic review analyzing current data on short and mid-term outcomes of TAVR in Patients with BAV stenosis. We also evaluated the impact of current generation balloon expandable prosthesis on outcomes in this patient population.
Full table
Methods
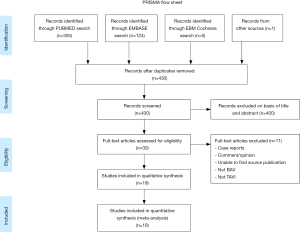
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews recommended by the Cochrane Collaboration was followed in this study (Figure 1). A systematic search via PubMed, Embase and Cochrane Database of all relevant reports published from 2002 to December 2018 including patients with BAV stenosis with or without counterparts with TAV stenosis that underwent TAVR were included. The search was limited to studies that were in English. A Boolean search was performed combining the following key words: ‘‘transcatheter aortic valve implantation’’ OR “transcatheter aortic valve replacement” AND “bicuspid aortic valve.” We manually scanned the bibliographies of included reports and relevant review articles to Identify additional studies. We did not include case reports or conference proceedings for unpublished or ongoing studies. Only studies reporting data on demographic and procedure characteristics, management, and clinical outcomes were included. Three authors (Tamunoinemi Bob-Manuel, Ikechukwu A. Ifedili, Mark R. Heckle) screened and retrieved reports, excluding irrelevant studies. Two authors (Uzoma N. Ibebuogu, Tamunoinemi Bob-Manuel) participated in the review process when there is uncertainty concerning the eligibility of a retrieved report. All publications were limited to those involving human subjects. BAV was confirmed by either multi-slice computed tomography (MSCT) and/or 2-dimensional echocardiography. Pre-procedural aortic annular measurement and sizing was defined as solely echocardiographic, MSCT only or both. We Included studies classified BAV according to the Sievers classification (18).
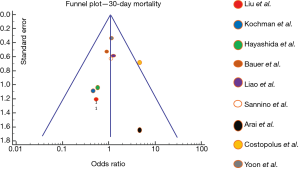
Outcomes including mortality, device success, PVL, and major vascular complications were defined according to the Valve Academic Research Consortium (VARC 2) (19). Post procedural PVL was considered significant if it was moderate or greater (≥3). Random-effect meta-analyses were performed for each outcome from all studies using the inverse variance method of Der Simonian and Laird (20). Odds ratio (OR) and 95% confidence interval (CI) were used to estimate post-TAVR outcome events. The heterogeneity of random effect estimates across the studies was assessed using I2 (21) (where I2<25%, low heterogeneity; I2=25% to 50%, moderate heterogeneity; and I2>50%, substantial heterogeneity). Publication bias was assessed with funnel plot of reported 30-day mortality in each study. Statistical analyses were performed using SPSS version 24 (IBM Corporation, Armonk, New York, USA).
Results
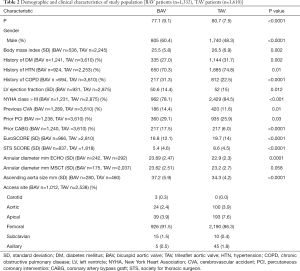
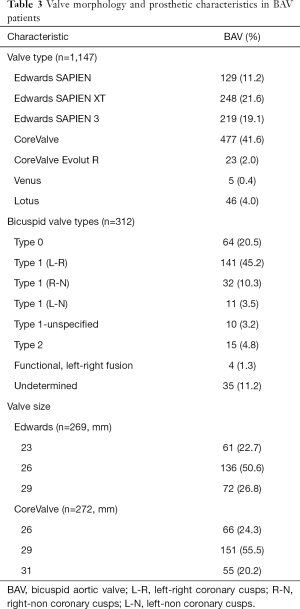
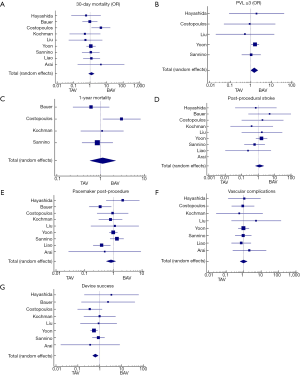
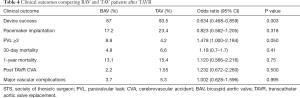
After duplicates were removed and exclusion criteria was applied, a total of 19 publications describing TAVR in BAV patients with or without TAV patients for comparison were included in our study. Totally, 1,332 patients (60.4% men, mean age of 77±9.1 years) with BAV and 3,610 patients (48.3% men, mean age 80±7.5 years) with TAV were analyzed. Of the 19 studies, 10 assessed outcomes in BAV alone while 9 assessed outcomes of BAV versus TAV patients. Baseline characteristics of included patients are summarized in Table 2. As expected, patients with BAV were significantly younger (P<0.0001), were more likely to be male (P<0.0001), had a lower STS score (P<0.0001) and had a significantly larger ascending aorta size (P<0.0001) (Table 2). Valve morphology and prosthesis characteristics are summarized in Table 3. There was no difference in 30-day mortality rate in patients with BAV compared with patients with TAV (OR: 1.18, 95% CI: 0.7–1.7, P=0.41, I2=0). There was no difference in 1-year mortality between patients with BAV compared with patients with TAV (OR: 1.12, 95% CI: 0.56–2.2). There was moderate heterogeneity in this analysis (I2=50%).
Full table
Full table
Device success was significantly lower in BAV patients (OR: 0.63, 95% CI: 0.49–0.86, P=0.003, I2=0) and moderate to severe PVL (≥3) was significantly higher in BAV patients (OR: 1.478, 95% CI: 1.000–2.184, P=0.050, I2=0).
Forest plots summarizing the outcomes of the study (Figure 2, Figures S1,S2) did not show publication bias for 30-day mortality (I2=0; significance level, P=0.58). There were no other significant differences in outcomes between both groups. Clinical outcomes comparing BAV and TAV in the study population are summarized in Table 4. When the analysis was restricted to the newer generation balloon expandable valve and the Lotus valve, the 30-day mortality rate and incidence of new pacemaker implantation rate was 3.7% and 24% respectively, with no moderate or severe PVL reported. Valve malposition occurred in 25 (5.3%) patients, out of whom 42.3% underwent a valve-in-valve TAVR, 38.5% were converted to a SAVR while 7.7% underwent balloon valvuloplasty. Major vascular complications defined as aortic dissections, major hemorrhage and major structural complications occurred in 5.9% of cases. When compared to the Balloon-Expandable Edwards and Edwards XT valves. The newer generation Edwards SAPIEN 3 and Lotus devices had much lower incidence of PVL compared to the older generation valve.
Full table
Discussion
TAVR procedural difficulty in BAV patients
A substantial proportion of patients with aortic stenosis have bicuspid valves, and surgery has been considered their only option because TAVR was relatively contraindicated (22) due to the difference in annular structure between BAV and trileaflet aortic valve (TAV), and uncertainty concerning the compatibility of current prosthesis with BAV (23). Prior relatively small studies have shown low mortality rates and a suggestion to perform TAVR in BAV patients (24,25). The short and mid-term outcomes of our relatively large cohort suggest that TAVR is safe and efficacious in BAV patients. Although we have proof of only short and medium-term benefit (30-day and 1-year mortality rates of 4.8% and 13.1% respectively), these findings are similar to those reported for patients with TAV (10). One of the main drawbacks in BAV patients from published TAVR studies is increased PVL due to increased calcium, its elliptical shape and dilated horizontal aorta (26), making it technically difficult to achieve optimal procedural results with a recent publication providing imaging and procedural insights on ways to tackle these challenges (27).
Comparison of outcomes in TAV versus BAV patients in our cohort
Nine out of 19 studies in our metanalysis contained TAV patients as a comparison cohort also undergoing TAVR (10,14,22,28-32). Hence, we performed a subanalysis comparing the post-TAVR outcomes in both groups (Figure S1). The TAV patients had better outcomes. However, the post-procedural outcomes of the BAV patients revealed acceptable complication rates, further advocating for this procedure in BAV patients. Mean age and STS scores were higher in the TAV group compared to the BAV group. This is likely because BAV patients present at a much younger age and have accelerated calcification leading to severe aortic stenosis, while the TAV patients were more likely to be older and have other medical comorbidities leading to higher STS scores. Mean age for the BAV patients although less than TAV patients was considerably advanced at 77±9.1 this can be accounted for by late diagnoses and TAVR implantation in these patients. Patients with moderate or severe PVL post-TAVR was higher in the BAV group compared to the TAV group (8.8% vs. 4.2%, P=0.05) however this is an acceptable post-procedural outcome in bicuspid patients and can be explained by the wider ascending aorta and aortic annulus. Higher incidence of valve malposition, PVL and lower device success is likely a consequence of these anatomic problems in bicuspid patients as mentioned above. There was no statistically significant difference in 30-day mortality or 1-year mortality between BAV and TAV There was no difference between pacemaker implantation rates, post-TAVR stroke and vascular complications between both groups, equally low in both growths.
Outcomes based on annular imaging modalities used for TAVR in BAV patients
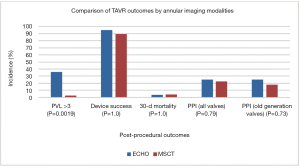
The exclusive use of echocardiography for annular measurement and device sizing was associated with a higher PVL rate compared to when device sizing is done with MSCT (2.8% vs. 36%, P=0.0019). This is likely because annular measurement with echocardiography is less accurate and results in device under-sizing and thus higher PVL. Hence CT is the imaging modality of choice for pre-TAVR planning according to the latest guidelines (4,5). Among BAV patients, Device success, 30-day mortality and permanent pacemaker implantation was similar regardless of imaging modality used (Figure 3).
Outcomes following TAVR with newer generation valves in BAV patients
Subanalysis of our data on outcomes in newer generation valves showed a robust reduction in PVL with a 37% incidence of mild PVL when BAV patients received the SAPIEN 3 Valve (33) and no moderate or severe PVL when they received the SAPIEN 3 or Lotus valves (14,33-35). In contrast, the self-expanding CoreValve trial reported a 7.8% incidence of moderate or severe PVL in TAV patients (36), while the Placement of Aortic Transcatheter Valves (PARTNER) trials using the older generation Edwards SAPIEN reported 12% incidence of moderate or severe PVL in TAV patients (1,2). Yoon et al. (14), the only study to include the new generation Self-Expandable valve Evolut R in BAV patients in our systematic review did not separately report PVL outcomes for this valve type. However, we can extrapolate from the CoreValve US Pivotal High-risk study (37) that the newer generation Evolut R will have comparable outcomes (30-day mortality and PVL) to the new generation SAPIEN 3.
The Edwards SAPIEN 3 valve with an outer sealing skirt, enhanced frame geometry for ultra-low delivery profile, high radial strength for circularity and optimal hemodynamics showed a reduction in PVL albeit with a slightly high pacemaker implantation rate (14,33,34).
The Lotus valve, another new generation valve, has been shown to have very low mortality and PVL (14,35) with full repositionability prior to release. The new catheter with reduced profile is designed to be more flexible and trackable and feature Depth Guard™ technology, designed to reduce LVOT interaction and PPM rate.
The observed high pacemaker implantation rate Post TAVR with the SAPIEN 3 valve (20%) (Table 4) may be due to the inclusion of very few patients and possibly other anatomic or procedural complications predisposing to pacemaker requirement along with the higher implantation height, which has been corrected in more recent studies using this valve (38). In summary, the newer generation prosthesis clearly reduces PVL, and with further advancement in prosthesis technology, PVL may eventually be eliminated in both BAV and TAV patients.
Future directions
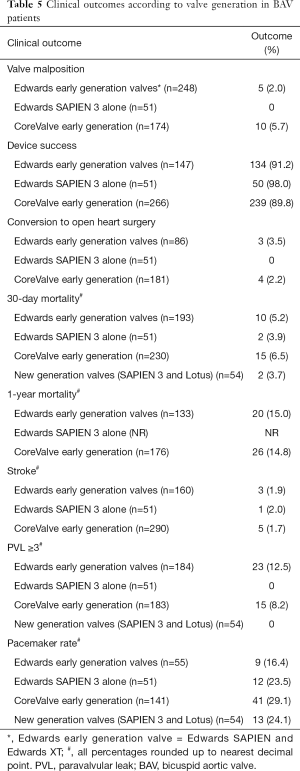
Taking the two main types of valves included in our study, there are subtle advantages and disadvantages with regards to TAVR in BAV patients (Table 5) although no direct comparison between the two different types of valves were made in the individual studies. This could be a topic of interest for future trials. Regardless of device type used for TAVR in BAV, the structural abnormalities associated with BAV such as enlarged aortic root, dilated ascending aorta, and functional aortic incompetence results in technical challenges in placing and deploying the chosen prosthesis successfully (39).
Full table
Conclusions
In our analysis, TAVR resulted in good one-year outcomes in BAV patients with severe aortic stenosis comparable to published data on TAVR in TAV. Also, the incidence of moderate to severe PVL that was significant with older generation device improved with newer generation device. Larger studies are needed to properly analyze and assess long- term structural and mortality outcomes associated with TAVR in this patient population before we can generalize results. TAVR was recently shown to be effective in Intermediate risk patients with trileaflet aortic stenosis (40) and it is anticipated that with younger TAVR population, the proportion of patients with bicuspid valves undergoing TAVR will be expected to increase leading to clinical and research interest in this subgroup of patients.
Our study unlike other metanalysis on BAV patients, is the first to analyze differences between newer and older generation valves, imaging modalities used for valve sizing and comparing BAV and TAV outcomes comprehensively.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200-54. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920-5. [Crossref] [PubMed]
- Zegdi R, Blanchard D, Azarine A. Elliptical shape of a SAPIEN XT prosthesis deployed in a patient with bicuspid aortic valve stenosis. J Heart valve Dis 2012;21:764-6. [PubMed]
- Zegdi R, Ciobotaru V, Noghin M, et al. Is it reasonable to treat all calcified stenotic aortic valves with a valved stent? Results from a human anatomic study in adults. J Am Coll Cardiol 2008;51:579-84. [Crossref] [PubMed]
- Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014;129:673-82. [Crossref] [PubMed]
- Costopoulos C, Latib A, Maisano F, et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol 2014;113:1390-3. [Crossref] [PubMed]
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789-800. [Crossref] [PubMed]
- Roberts WC, Janning KG, Ko JM. Frequency of congenitally bicuspid aortic valves in patients >/=80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol 2012;109:1632-6. [Crossref] [PubMed]
- Yoon SH, Lefèvre T, Ahn JM, et al. Transcatheter Aortic Valve Replacement With Early- and New-Generation Devices in Bicuspid Aortic Valve Stenosis. J Am Coll Cardiol 2016;68:1195-205. [Crossref] [PubMed]
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol 2017;69:2579-89. [Crossref] [PubMed]
- Nagaraja V, Suh W, Fischman DL, et al. Transcatheter aortic valve replacement outcomes in bicuspid compared to trileaflet aortic valves. Cardiovasc Revasc Med 2019;20:50-6. [Crossref] [PubMed]
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: Systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018;91:975-83. [Crossref] [PubMed]
- Kanjanahattakij N, Horn B, Vutthikraivit W, et al. Comparing outcomes after transcatheter aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve: A systematic review and meta-analysis. Clin Cardiol 2018;41:896-902. [Crossref] [PubMed]
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105-14. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Bauer T, Linke A, Sievert H, et al. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). Am J Cardiol 2014;113:518-21. [Crossref] [PubMed]
- Kochman J, Huczek Z, Scislo P, et al. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol 2014;114:757-62. [Crossref] [PubMed]
- Phan K, Wong S, Phan S, et al. Transcatheter Aortic Valve Implantation (TAVI) in Patients with Bicuspid Aortic Valve Stenosis—Systematic Review and Meta-Analysis. Heart Lung Circ 2015;24:649-59. [Crossref] [PubMed]
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014;64:2330-9. [Crossref] [PubMed]
- Philip F, Faza NN, Schoenhagen P, et al. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv 2015;86:E88-98. [Crossref] [PubMed]
- Frangieh AH, Kasel AM. TAVI in Bicuspid Aortic Valves ‘Made Easy’. Eur Heart J 2017;38:1177-81. [Crossref] [PubMed]
- Hayashida K, Bouvier E, Lefèvre T, et al. Transcatheter Aortic Valve Implantation for Patients With Severe Bicuspid Aortic Valve Stenosis. Circ Cardiovasc Interv 2013;6:284-91. [Crossref] [PubMed]
- Liu XB, Jiang JB, Zhou QJ, et al. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B 2015;16:208-14. [Crossref] [PubMed]
- Arai T, Lefèvre T, Hovasse T, et al. The feasibility of transcatheter aortic valve implantation using the Edwards SAPIEN 3 for patients with severe bicuspid aortic stenosis. J Cardiol 2017;70:220-4. [Crossref] [PubMed]
- Liao YB, Li YJ, Xiong TY, et al. Comparison of procedural, clinical and valve performance results of transcatheter aortic valve replacement in patients with bicuspid versus tricuspid aortic stenosis. Int J Cardiol 2018;254:69-74. [Crossref] [PubMed]
- Sannino A, Cedars A, Stoler RC, et al. Comparison of Efficacy and Safety of Transcatheter Aortic Valve Implantation in Patients with Bicuspid Versus Tricuspid Aortic Valves. Am J Cardiol 2017;120:1601-6. [Crossref] [PubMed]
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid Aortic Valve Stenosis: Favorable Early Outcomes with a Next-Generation Transcatheter Heart Valve in a Multicenter Study. JACC: Cardiovasc Interv 2016;9:817-24. [Crossref] [PubMed]
- Jilaihawi H, Chen M, Webb J, et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC: Cardiovasc Imaging 2016;9:1145-58. [Crossref] [PubMed]
- Chan AW, Wong D, Charania J, et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Stenosis Using Lotus Valve System. Catheter Cardiovasc Interv 2017;90:157-63. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic- valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Reardon MJ, Adams DH, Kleiman NS, et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113-21. [Crossref] [PubMed]
- De Torres-Alba F, Kaleschke G, Diller GP, et al. Changes in the Pacemaker Rate After Transition from Edwards SAPIEN XT to SAPIEN 3 Transcatheter Aortic Valve Implantation: The Critical Role of Valve Implantation Height. JACC Cardiovasc Interv 2016;9:805-13. [Crossref] [PubMed]
- Napodano M, Tarantini G, Ramondo A. Is it reasonable to treat all calcified stenotic valves with a valve stent? Probably yes if we get a full stent expansion. J Am Coll Cardiol 2009;53:219. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]