
Total en bloc resection of primary and metastatic spine tumors
Introduction
En bloc resection involves the surgical removal of the entirety of a tumor without violating its capsule, and requires resection of the lesion encased by a continuous margin of healthy tissue. An en bloc approach for tumors of the spine is accompanied by a unique set of anatomical considerations, and requires adaptation of surgical principles from both appendicular musculoskeletal and neurosurgical oncology. The en bloc technique was first coined by Enneking et al., within the context of primary musculoskeletal sarcoma (1). In their description of a surgical staging system, the authors contrast intralesional resection—consisting of piecemeal debulking or curettage—against en bloc resections, with either marginal, wide, or radical resection of the tumor along with varying margins of normal tissue.
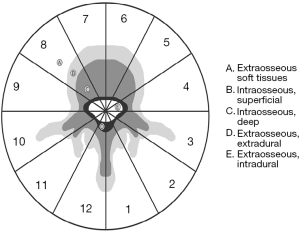
Boriani et al. adapted the Enneking staging system for application in tumors of the spine. Their novel classification and staging of spinal tumors led to the Weinstein-Boriani-Biagini (WBB) staging system (Figure 1) (2). This system delineates 12 radiating zones in the axial plane of the vertebral body, five concentric layers of tumor involvement surrounding the dural sac, and accounts for the number of vertebral levels involved (2). Gross and histologic evaluation can further classify the operation as “intralesional” if the mass has been cut, “marginal” if the pseudocapsule has been dissected out, and “wide” if the tumor has been removed with a continuous margin of normal tissue (2). This system accepts that “radical” margins are unattainable in spinal lesions contiguous with the epidural space whilst creating a platform from which to plan complete resection without tumor breach. The WBB system has been clinically validated (3) and seems to accurately predict intraoperative margins in a majority of patients (4). This classification system lends to more informed preoperative planning and allows for total en bloc resections to be performed in a growing number of cases (5).
The term “total en bloc spondylectomy” was first coined by Tomita et al. Their description of this procedure specifically entailed an en bloc laminectomy and posterior instrumentation followed by a total vertebrectomy and anterior column reconstruction for tumors contained solely within the vertebral body (6). Since its first description, this technique has been increasingly employed with successful outcomes in a variety of patient populations (4,5,7-11). Generally, en bloc resections are appropriate in patients whose lesions do not invade adjacent viscera nor adhere to adjacent major blood vessels (12), and thus require careful patient selection and surgical planning.
En bloc resection in the spine is generally indicated for malignant primary tumors as well as aggressive benign tumors (1,4). This technique can also be employed for amenable solitary metastatic lesions including hormone secreting and radio-insensitive tumors (13-16). However, en bloc resection often involves a technically demanding procedure, and carries a high rate of complications. Improvements in tumor-related mortality must be balanced against procedure-related morbidity, and local lesion control against preservation of function (1,8). Due to the relative complexity of this surgical procedure, much of the en bloc literature consists of case reports and series. This review seeks to summarize the existing body of evidence regarding the indications and utility of en bloc resection of primary and metastatic spinal tumors.
Survival & recurrence
In keeping with oncologic principles, removal of the tumor in its entirety without violation of the capsule should confer lower rates of future recurrence. Several studies have borne this out, showing recurrence rates to be higher in intralesional than en bloc surgeries (4,12,17-20). Furthermore, marginal en bloc resections are at higher risk of recurrence than wide en bloc resections (4). However, it is worth noting that wide resections are often unattainable in the spine, owing to its anatomical complexity.
Successful en bloc resection appears to especially improve recurrence-free and overall survival for aggressive primary tumors (4,21). A recent review estimated disease-free survival following en bloc resection to be 92.6%, 63.2%, and 43.9% at 1, 5, and 10 years, respectively, in a primary tumor cohort (5). Ten-year overall survival in the same cohort was estimated to be 71%, and 5-year survival was assessed to be 84.4% (5,22). However, it is worth noting that within this category there exists significant variability in baseline expected survival, recurrence rates and surgical morbidity, and any improvement following en bloc resection must be considered within the context of the primary pathology. Survival and recurrence rates by tumor type are detailed in a later section of this review.
Similarly, prognosis for metastatic lesions following en bloc resection varies widely based on primary pathology and systemic disease status. A recent review in this population estimated disease-free survival at 1, 5, and 10 years to be 61.8%, 37.5%, and 0%, respectively (5). Overall survival was undefined for that cohort, but mean survival has been estimated by other studies to range between 15 and 27 months (23,24). Local recurrence rates following en bloc resection for metastases have been reported to be as low as 11% (16).
For both primary and metastatic lesions, prior radiation therapy has been identified as a risk factor for local recurrence (16). This may be due to radiation-related changes to the peritumoral tissue, which can lead to indiscriminate tumor boundaries. Intraoperative dural tear and tumor occupancy rate of >50% of the spinal canal also predict future local recurrence (16). Unsurprisingly, recurrence rates have been reported to be higher in cases of reoperation, and if performed at a non-tertiary center (4,25).
Complications
Despite the benefits of en bloc resection in appropriately selected patients, this demanding technique nonetheless has significant risks (26). The high morbidity of spinal oncologic resections in general has been previously well-described (27-29). A demarcation zone between neoplastic and healthy tissue can be evasive or nonexistent, further complicating this technique. The potential also exists for tumor cell contamination of the surrounding structures during resection (12). Overall, en bloc resection carries an increased complication rate when compared with intralesional resection (8). Institutions who have more recently begun performing en bloc resections have published complication rates as high as 76% (23), highlighting the importance of surgeon comfortability with undertaking this procedure.
Several operative factors have been shown to affect complication rates. Anterior-posterior combined approaches independently increase the incidence of both major and minor complications as compared to a posterior-only approach (4,8). This finding is unsurprising given the likelihood that a combined procedure be used in cases with more complex anatomical involvement, and may have higher associated blood loss and procedural morbidity. Prior surgery or open biopsy also appears to confer increased risk of major complications as delineated by the McDonnell classification (4,8,30). Prior radiation therapy increases rates of infection, but not overall complication rate (8). Increased number of levels also increases risk of complications (4).
Primary spine tumors
Primary spine tumors account for 11% of primary musculoskeletal tumors, less than 5% of all spinal tumors, and only roughly 0.4% of all malignancies (14). En bloc resection has proven effective in improving prognosis and decreasing local recurrence for primary aggressive spinal lesions (12,20,31). Amendola et al. recently performed a prospective cohort study of 103 patients who underwent en bloc resection of primary benign and malignant spine lesions (4). En bloc resection was associated with decreased risk of local recurrence and overall tumor-related mortality. At mean follow-up of 100 months, 69 patients (67.0%) showed no evidence of recurrence. Of note, the risk of local recurrence following en bloc resection was significantly higher in patients who previously underwent an operation—either intralesional excision or open biopsy—than patients with no prior surgery [hazard ratio (HR) =3.45, 95% CI, 1.38–8.63]. Forty-three patients (41.7%) presented with a total of 75 postoperative complications; in accordance with the McDonnell classification, 40 major and 35 minor complications were observed (4). Smaller case series have borne out similarly positive results (12).
With respect to specific primary tumor types, the role of en bloc resection has largely been investigated via case series and reports. Chordomas are slow-growing yet malignant primary bony lesions that can affect the spine. En bloc resection can offer a surgical technique for controlling locally aggressive chordomas, or those that threaten nearby viscera or vasculature. A 2018 systematic review concluded that en bloc excision remains the gold standard for the management of chordomas, and emphasized the importance of multimodal adjuvant therapy for these tumors (32). Recent analysis of the AOSpine Knowledge Forum Tumor database showed improved overall survival (8.4 vs. 6.4 years, P=0.023) in patients resected in keeping with Enneking principles, as compared to Enneking-inappropriate patients. Additionally, Enneking-inappropriate resection conferred a greater risk of local recurrence (HR, 7.02, 95% CI: 2.96–16.6, P<0.001) (17).
Chondrosarcoma is a malignant osseous neoplasm that accounts for only 10% of all primary bone tumors (14). These tumors are rare in the axial skeleton, with an estimated 2–12% of all chondrosarcomas arising in the spinal column (21). Chondrosarcoma of the spine is notoriously difficult to treat and has proven resistant to both radiation therapy and chemotherapy (9,33,34). Surgical resection correlates with overall survival benefit, and en bloc resection has been touted as the optimal surgical option for these lesions (9,14,35-38). Notably, a recent analysis of the Surveillance, Epidemiology, and End Results Registry (SEER) registry analyzed 973 cases of chondrosarcoma of the spine (37). Of these cases, the surgical cohort demonstrated overall and disease-free survival benefits over both the radiotherapy alone and adjuvant radiotherapy cohorts. Importantly, this review did not explicitly analyze extent of resection, due to limitations of the surgical data available via the SEER database (37). Ambispective cohort analysis of 111 patients with primary spinal chondrosarcoma demonstrated an improvement in local recurrence rates following Enneking appropriate resection (18). Finally, a recent review of 84 cases of primary chondrosarcoma of the spine calculated patients receiving non-en bloc resection to have a 9.4 times hazards ratio for death compared with those receiving an en bloc resection (95% CI: 2.6–34, P=0.001) (39).
En bloc resection can also provide local control for several subtypes of sarcoma occurring in the spine. Recent analysis of 58 patients who underwent surgical treatment for primary spinal osteosarcoma demonstrated that en bloc resection in accordance with Enneking principles conferred increased survival and decreased local recurrence over intralesional resection (38). Promising results have also been reported following en bloc resection of synovial sarcomas—a soft tissue tumor with reported cases in the axial skeleton—though these results are limited to case reports and series (40-42).
Giant cell tumors (GCTs) comprise another primary bone tumor for which en bloc resection may be employed. These tumors often occur in the vertebral body, and 1.4–9.4% occur above the sacrum, in the mobile spine (25). Boriani et al. found that en bloc resection conferred a survival benefit: average time to local recurrence following en bloc excision was 197 months, versus 91 months in an intralesional excision cohort (P=0.03) (25). Recurrence rates are higher in GCTs involving the posterior elements, as well as in lesions with extra-osseus or paraspinal extension (43). Recent analysis of the AOSpine Knowledge Forum Tumor database supported these findings: en bloc resection with wide margins conferred a significantly reduced likelihood of local recurrence as compared to intralesional resection (P=0.029) (19). Some authors have suggested that preoperative denosumab may further facilitate the feasibility of complete resection of GCTs (44-46).
Metastatic spine tumors
Spinal metastases represent the most common type of spine tumor, occurring over 20 times more frequently than primary spinal neoplasms (47). The spine is the most common site for skeletal metastasis, with lesions of the axial skeleton representing roughly 39% of all bony metastases (27). Cord compression secondary to spinal metastases occurs in 5–10% of all cancer patients, and up to 40% of patients with existing nonspinal bone metastases (48). Spinal metastases subsequently represent a significant source of pain and disability, and a potential opportunity for surgical intervention and improvement of quality-of-life. Despite the prevalence of spinal metastases, there exists a paucity of data assessing the efficacy of en bloc resection in this patient population.
Breast, prostate, and lung cancers classically represent the most common primary tumors with propensity to metastasize to the bony spine (49). En bloc resection has been reported to be an appropriate surgical option with appropriate patient selection and favorable status of systemic disease (13-15,50). However, systemic burden of oncologic disease often determines morbidity and mortality in this population. Therefore, the benefits of an aggressive en bloc resection technique may not always outweigh the risks, and consideration of all patient characteristics is imperative in determining optimal extent of resection.
A recent analysis of 91 patients who underwent en bloc resection for metastatic spine lesions demonstrated a local recurrence rate of 11%, at a mean follow-up duration of 27.4 months (range, 4–66 months) (16). A history of prior radiotherapy (P=0.04), intraoperative dural tear (P=0.03), and tumor occupancy rate of >50% of the spinal canal (P=0.02) were found to be predictive of future local recurrence. Sakaura et al. studied twelve patients who underwent en bloc resection of solitary thoracic metastases. En bloc resection provided long-term control to several patients within their cohort, with seven patients surviving for an average of 61 months (10). Similarly, Huang et al. recently reported outcomes of nine en bloc resections for solitary metastases to the lumbar spine (51). Five patients remained disease-free at the time of most recent follow-up (mean follow-up 41.2 months). Another analysis demonstrated average survival following en bloc resection to be 15 months for patients with metastatic lesions, compared to 47.6 months in patients with primary spinal tumors (23). The benefits of en bloc resection, therefore, may be less dramatic in a metastatic population, but remain non-negligible.
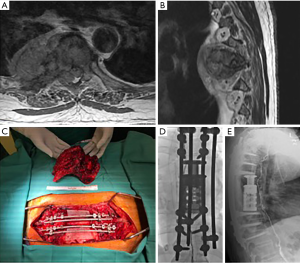
En bloc resection may provide particular benefit to patients with radioresistant metastases. Classically, renal, hepatocellular, colon, thyroid, and non-small cell lung carcinomas and melanoma are considered to be less responsive to radiation therapy (52) (Figure 2). Even in an era of increasingly precise radiosurgery, the epidural space and spinal cord represent important dose-constraining structures, and therapeutic doses to these tumor types may not be attainable in the spine. Without radiation as a useful adjunct for these lesions, en bloc resection may provide a better chance at local control than a more conservative resection. Case series have previously described successful en bloc removal of renal cell, non-small cell, thyroid, and hepatocellular carcinomas (24,53-56).
In the case of patients with secretory spinal metastases, en bloc resection can provide a uniquely useful surgical option. This technique has been described in the setting of pheochromocytoma, carcinoid, paraganglioma, choriocarcinoma, and fibroblast growth factor 23-secreting phosphaturic mesenchymal tumors (15,57-59). These tumor types may uniquely benefit from en bloc resection, as this approach theoretically eliminates the functional ability of the lesions and provides improved symptom control over a subtotal resection (60).
Finally, several rare tumor types have been demonstrated to be amenable to en bloc resection. Cases have been reported of optimal long-term outcomes in metastatic epithelial-myoepithelial carcinoma (61), renal cell carcinoma of the spine (24), leiomyosarcoma (62), metastatic osteosarcoma (63), and acinic cell carcinoma (64), among others. However, due to the rare nature of these tumor types in the context of spinal metastases, little can be definitively concluded about the overall superiority of en bloc resection in these cases.
Surgical technique and anatomic considerations
Single posterior approach
A single posterior approach can be an appealing surgical option in patients with concurrent comorbidities that preclude more extensive or staged procedures (65). This approach can also be employed in cases of prior surgery, prior radiotherapy, or unresectable anterior paraspinal tumor or scar tissue (12,65,66). A posterior-only approach may provide an ideal option for especially muscular or obese patient populations (67). Posterior approach involves excision of the posterior elements, which allows hemostasis of the epidural venous plexus, sectioning of the posterior longitudinal ligament and annulus fibrosis, as well as posterior stabilization (2). A major disadvantage to this technique is a lack of direct visualization of ventral structures (5).
Combined posterior anterior-posterior approach
A combined anterior and posterior approach can increase the likelihood of obtaining an en bloc resection without violation of the tumor capsule, but poses additional challenges and necessitates greater coordination of surgical resources and skill. A combined spondylectomy may be performed as staged or simultaneous procedures. A non-staged approach allows for simultaneous anterior and posterior stabilization, but also demands the technical ability to achieve posterior reconstruction with the patient in lateral decubitus (5,68).
Cervical
The location of a lesion along the spinal column can drastically affect the surgical approach and feasibility of an en bloc resection. Employing this surgical technique in the cervical spine presents a unique set of considerations. The presence of the vertebral arteries within the transverse foramina poses an anatomical challenge to resection. If en bloc resection cannot be achieved while preserving bilateral vertebral arteries, a balloon-test occlusion can assess the collateral capacity of the Circle of Willis and the safety of sacrificing one vertebral artery (69). Digital-subtraction angiogram of the cerebral vessels can also assess the safety of vessel sacrifice (70). Endovascular vertebral artery occlusion may be performed prior to surgical ligation (71), though many authors recommend occluding after posterior instrumentation due to the potential risk of injury to the contralateral artery.
Posterior, anterolateral, retropharyngeal, and lateral en bloc approaches to tumors of the cervical spine have previously been described (72,73). A combined transmaxillary and transmandibular en bloc approach has also been described for a C1 chordoma (74). Hsieh et al. detail a staged procedure for cervical chordoma resection, with the first stage comprised of release osteotomies, posterior tumor dissection, placement of instrumentation, posterolateral arthrodesis, and any required nerve root or vessel sacrifice, followed 2–5 days later by anterior dissection and en bloc resection (70).
Thoracic
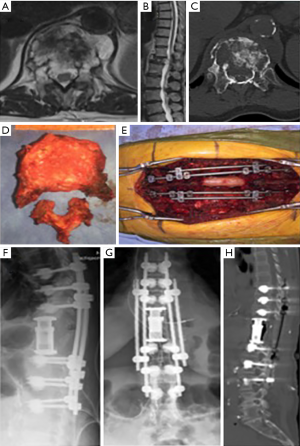
The thoracic spine poses yet another set of distinct surgical considerations. Anterior access can be limited by the proximity of the esophagus and great vessels (75). The ribs and mediastinal structures may further impede thoracic access. Thoracic approaches often necessitate blunt dissection of the pleura from the ribs and vertebrae, and may require ligation of the intercostal vessels. Techniques of anterior, posterior, anterior followed by posterior, and posterior followed by anterior, have been identified and proposed for en bloc resection (75-79). A combined approach can facilitate dissection of the aortic branches, and may be preferable in cases involving the great vessels or at junctional locations. Sciubba et al. achieved en bloc resection of a T1–5 chordoma using a 5-level spondylectomy and bilateral chest wall resection, from which the patient recovered without neurologic complication (77). A simultaneous thoracoscopic and posterior approach has also been described to safely achieve resection of a T2–3 chordoma with paravertebral involvement (75). A similar technique has been used for resection of a T11 metastasis and a T5–6 osteogenic sarcoma (80) (Figure 3).
Lumbar
When en bloc resection is employed in the lumbar spine, additional care must be taken due to the anatomical proximity of the lumbar plexus and bowel, the vascular lumbar pedicles, and muscular insertions at the vertebral bodies. Some authors have suggested that a staged procedure is subsequently required to avoid complications (22,81). The utility of a single posterior approach is limited in the lumbar spine, due to the muscular insertions at the vertebral bodies (5). Still, some have suggested that a posterior-only approach is reasonable at the L3–5 levels (82).
Sacral
Sacral tumors that lend themselves to en bloc resection can either be approached via a combined or posterior-only approach. Anterior access allows for mobilization and preservation of critical structures such as the rectum and internal iliac vessels. This approach also allows the surgeon to obtain a vascularized flap to aid in the closure of the posterior component. The combined anterior-posterior approach has been well-described (83-85). However, a posterior-only approach has been used by several groups, who emphasize the decreased morbidity of reducing without sacrificing en bloc principles (86-88).
Conclusions
- En bloc resection improves local control over intralesional resection for primary aggressive lesions (4,12,20,21,31). The associated improvement in overall survival may warrant the increased morbidity of surgery, as these tumor types are traditionally poor responders to adjuvant treatment options.
- En bloc may be an appropriate technique for carefully selected metastatic lesions—such as hormone-secreting tumors and solitary radioresistant tumors—but must be considered in the context of the patient’s systemic disease status and the morbidity of surgery (13-15,50).
- Complication rates are higher following en bloc resection as compared to conventional resection techniques (76).
- Patients being considered for en bloc resection may be best managed by specialized surgeons in high-volume tertiary centers (23,25).
Acknowledgments
None.
Footnote
Conflicts of Interest: IO Karikari, MD; Consultant for Nuvasive, Depuy. Receives spine fellowship fund from Nuvasive; M Abd-El-Barr, MD, PhD: Consultant for Spineology; ML Goodwin, MD, PhD: Consultant for ROM3 Rehab, Augmedics; DM Sciubba, MD: Consultant for Orthofix, Globus, K2M, Medtronic, Stryker, Baxter; CR Goodwin, MD, PhD: Received grants from the Burroughs Wellcome Fund, North Carolina Spine Society, and the NIH/NINDS K12 NRCDP Physician Scientist Award, Robert Wood Johnson Harold Amos Medical Faculty Development Program. The other authors have no conflicts of interest to declare.
References
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980.106-20. [PubMed]
- Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976) 1997;22:1036-44. [Crossref] [PubMed]
- Fisher CG, Keynan O, Boyd MC, et al. The surgical management of primary tumors of the spine: initial results of an ongoing prospective cohort study. Spine (Phila Pa 1976) 2005;30:1899-908. [Crossref] [PubMed]
- Amendola L, Cappuccio M, De Iure F, et al. En bloc resections for primary spinal tumors in 20 years of experience: effectiveness and safety. Spine J 2014;14:2608-17. [Crossref] [PubMed]
- Cloyd JM, Acosta FL Jr, Polley MY, et al. En bloc resection for primary and metastatic tumors of the spine: a systematic review of the literature. Neurosurgery 2010;67:435-44; discussion 444-5. [Crossref] [PubMed]
- Tomita K, Toribatake Y, Kawahara N, et al. Total en bloc spondylectomy and circumspinal decompression for solitary spinal metastasis. Paraplegia 1994;32:36-46. [PubMed]
- Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976) 2006;31:493-503. [Crossref] [PubMed]
- Boriani S, Bandiera S, Donthineni R, et al. Morbidity of en bloc resections in the spine. Eur Spine J 2010;19:231-41. [Crossref] [PubMed]
- Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000;25:804-12. [Crossref] [PubMed]
- Sakaura H, Hosono N, Mukai Y, et al. Outcome of total en bloc spondylectomy for solitary metastasis of the thoracolumbar spine. J Spinal Disord Tech 2004;17:297-300. [Crossref] [PubMed]
- Stener B, Henriksson C, Johansson S, et al. Surgical removal of bone and muscle metastases of renal cancer. Acta Orthop Scand 1984;55:491-500. [Crossref] [PubMed]
- Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine (Phila Pa 1976) 1997;22:324-33. [Crossref] [PubMed]
- Sundaresan N, Boriani S, Rothman A, et al. Tumors of the osseous spine. J Neurooncol 2004;69:273-90. [Crossref] [PubMed]
- Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am 2009;40:21-36. v. [Crossref] [PubMed]
- Kaloostian PE, Zadnik PL, Awad AJ, et al. En bloc resection of a pheochromocytoma metastatic to the spine for local tumor control and for treatment of chronic catecholamine release and related hypertension. J Neurosurg Spine 2013;18:611-6. [Crossref] [PubMed]
- Igarashi T, Murakami H, Demura S, et al. Risk factors for local recurrence after total en bloc spondylectomy for metastatic spinal tumors: A retrospective study. J Orthop Sci 2018;23:459-63. [Crossref] [PubMed]
- Gokaslan ZL, Zadnik PL, Sciubba DM, et al. Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine 2016;24:644-51. [Crossref] [PubMed]
- Fisher CG, Versteeg AL, Dea N, et al. Surgical Management of Spinal Chondrosarcomas. Spine (Phila Pa 1976) 2016;41:678-85. [Crossref] [PubMed]
- Charest-Morin R, Fisher CG, Varga PP, et al. En Bloc Resection Versus Intralesional Surgery in the Treatment of Giant Cell Tumor of the Spine. Spine (Phila Pa 1976) 2017;42:1383-90. [Crossref] [PubMed]
- Stener B. Complete removal of vertebrae for extirpation of tumors. A 20-year experience. Clin Orthop Relat Res 1989.72-82. [PubMed]
- Katonis P, Alpantaki K, Michail K, et al. Spinal chondrosarcoma: a review. Sarcoma 2011;2011:378957. [Crossref] [PubMed]
- Sciubba DM, De la Garza Ramos R, Goodwin CR, et al. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur Spine J 2016;25:4080-7. [Crossref] [PubMed]
- Araujo AO, Narazaki DK, Teixeira WGJ, et al. En bloc vertebrectomy for the treatment of spinal lesions. Five years of experience in a single institution: a case series. Clinics (Sao Paulo) 2018;73:e95. [Crossref] [PubMed]
- Mosele GR, Caggiari G, Scarpa RM, et al. The treatment of vertebral metastases from renal cell carcinoma: a retrospective study. Minerva Urol Nefrol 2017;69:166-72. [PubMed]
- Boriani S, Bandiera S, Casadei R, et al. Giant cell tumor of the mobile spine: a review of 49 cases. Spine (Phila Pa 1976) 2012;37:E37-45. [Crossref] [PubMed]
- Boriani S, Gasbarrini A, Bandiera S, et al. En Bloc Resections in the Spine: The Experience of 220 Patients During 25 Years. World Neurosurg 2017;98:217-29. [Crossref] [PubMed]
- Wise JJ, Fischgrund JS, Herkowitz HN, et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1999;24:1943-51. [Crossref] [PubMed]
- Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976) 1995;20:1592-9. [Crossref] [PubMed]
- Pascal-Moussellard H, Broc G, Pointillart V, et al. Complications of vertebral metastasis surgery. Eur Spine J 1998;7:438-44. [Crossref] [PubMed]
- McDonnell MF, Glassman SD, Dimar JR 2nd, et al. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am 1996;78:839-47. [Crossref] [PubMed]
- Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine (Phila Pa 1976) 1996;21:1927-31. [Crossref] [PubMed]
- Denaro L, Berton A, Ciuffreda M, et al. Surgical management of chordoma: A systematic review. J Spinal Cord Med 2018.1-16. [Crossref] [PubMed]
- Ropper AE, Cahill KS, Hanna JW, et al. Primary vertebral tumors: a review of epidemiologic, histological and imaging findings, part II: locally aggressive and malignant tumors. Neurosurgery 2012;70:211-9; discussion 219. [Crossref] [PubMed]
- Strike SA, McCarthy EF. Chondrosarcoma of the spine: a series of 16 cases and a review of the literature. Iowa Orthop J 2011;31:154-9. [PubMed]
- Tessitore E, Burkhardt K, Payer M. Primary clear-cell chondrosarcoma of the cervical spine. Case illustration. J Neurosurg Spine 2006;4:424. [Crossref] [PubMed]
- Bergh P, Gunterberg B, Meis-Kindblom JM, et al. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer 2001;91:1201-12. [Crossref] [PubMed]
- Arshi A, Sharim J, Park DY, et al. Chondrosarcoma of the Osseous Spine: An Analysis of Epidemiology, Patient Outcomes, and Prognostic Factors Using the SEER Registry From 1973 to 2012. Spine (Phila Pa 1976) 2017;42:644-52. [Crossref] [PubMed]
- Dekutoski MB, Clarke MJ, Rose P, et al. Osteosarcoma of the spine: prognostic variables for local recurrence and overall survival, a multicenter ambispective study. J Neurosurg Spine 2016;25:59-68. [Crossref] [PubMed]
- Nisson PL, Berger GK, James WS, et al. Surgical Techniques and Associated Outcomes of Primary Chondrosarcoma of the Spine. World Neurosurg 2018;119:e32-45. [Crossref] [PubMed]
- Kim J, Lee SH, Choi YL, et al. Synovial sarcoma of the spine: a case involving paraspinal muscle with extensive calcification and the surgical consideration in treatment. Eur Spine J 2014;23:27-31. [Crossref] [PubMed]
- Cao Y, Jiang C, Chen Z, et al. A rare synovial sarcoma of the spine in the thoracic vertebral body. Eur Spine J 2014;23 Suppl 2:228-35. [Crossref] [PubMed]
- Yang M, Zhong N, Zhao C, et al. Surgical management and outcome of synovial sarcoma in the spine. World J Surg Oncol 2018;16:175. [Crossref] [PubMed]
- Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine (Phila Pa 1976) 1997;22:1773-82; discussion 1783.
- Inoue G, Imura T, Miyagi M, et al. Total en bloc spondylectomy of the eleventh thoracic vertebra following denosumab therapy for the treatment of a giant cell tumor. Oncol Lett 2017;14:4005-10. [Crossref] [PubMed]
- Agarwal A, Larsen BT, Buadu LD, et al. Denosumab chemotherapy for recurrent giant-cell tumor of bone: a case report of neoadjuvant use enabling complete surgical resection. Case Rep Oncol Med 2013;2013:496351. [Crossref] [PubMed]
- Goldschlager T, Dea N, Boyd M, et al. Giant cell tumors of the spine: has denosumab changed the treatment paradigm? J Neurosurg Spine 2015;22:526-33. [Crossref] [PubMed]
- Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am 2004;15:365-73. [Crossref] [PubMed]
- Schmidt MH, Fourney DR, Gokaslan ZL. Metastatic spine disease. Neurosurg Clin N Am 2004;15:xv-xvi. [Crossref] [PubMed]
- Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg 1983;59:111-8. [Crossref] [PubMed]
- Mesfin A, El Dafrawy MH, Jain A, et al. Total En Bloc Spondylectomy for Primary and Metastatic Spine Tumors. Orthopedics 2015;38:e995-1000. [Crossref] [PubMed]
- Huang W, Wei H, Cai W, et al. Total en bloc spondylectomy for solitary metastatic tumors of the fourth lumbar spine in a posterior-only approach. World Neurosurg 2018;120:e8-16. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Demura S, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal metastases in thyroid carcinoma. J Neurosurg Spine 2011;14:172-6. [Crossref] [PubMed]
- Matsumoto M, Tsuji T, Iwanami A, et al. Total en bloc spondylectomy for spinal metastasis of differentiated thyroid cancers: a long-term follow-up. J Spinal Disord Tech 2013;26:E137-42. [Crossref] [PubMed]
- Kimura H, Fujibayashi S, Shimizu T, et al. Successful Total En Bloc Spondylectomy of T7 Vertebra for Hepatocellular Carcinoma Metastasis After Living Donor Liver Transplantation. Spine (Phila Pa 1976) 2015;40:E944-7. [Crossref] [PubMed]
- Fadel E, Missenard G, Court C, et al. Long-term outcomes of en bloc resection of non-small cell lung cancer invading the thoracic inlet and spine. Ann Thorac Surg 2011;92:1024-30; discussion 1030. [Crossref] [PubMed]
- Basuroy R, Srirajaskanthan R, Prachalias A, et al. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther 2014;39:1071-84. [Crossref] [PubMed]
- Naito Y, Akeda K, Kasai Y, et al. Lumbar metastasis of choriocarcinoma. Spine (Phila Pa 1976) 2009;34:E538-43. [Crossref] [PubMed]
- Jia Q, Yin H, Yang J, et al. Treatment and outcome of metastatic paraganglioma of the spine. Eur Spine J 2018;27:859-67. [Crossref] [PubMed]
- Goodwin CR, Clarke MJ, Gokaslan ZL, et al. En Bloc Resection of Solitary Functional Secreting Spinal Metastasis. Global Spine J 2016;6:277-83. [Crossref] [PubMed]
- Goodwin CR, Khattab MH, Sankey EW, et al. Epithelial-myoepithelial carcinoma metastasis to the thoracic spine. J Clin Neurosci 2016;24:143-6. [Crossref] [PubMed]
- Ramirez-Villaescusa J, Canosa-Fernandez A, Martin-Benlloch A, et al. Free disease long-term survival in primary thoracic spine leiomyosarcoma after total en bloc spondylectomy: A case report. Int J Surg Case Rep 2017;39:332-8. [Crossref] [PubMed]
- Gupta S, Stinson ZS, Marco RA, et al. Single stage en bloc resection of a recurrent metastatic osteosarcoma of the pediatric lumbar spine through multiple exposures - a novel approach. Sicot J 2018;4:32. [Crossref] [PubMed]
- Sangsin A, Murakami H, Shimizu T, et al. Four-Year Survival of a Patient With Spinal Metastatic Acinic Cell Carcinoma After a Total En Bloc Spondylectomy and Reconstruction With a Frozen Tumor-Bearing Bone Graft. Orthopedics 2018;41:e727-30. [Crossref] [PubMed]
- Casadei R, Mavrogenis AF, De Paolis M, et al. Two-stage, combined, three-level en bloc spondylectomy for a recurrent post-radiation sarcoma of the lumbar spine. Eur J Orthop Surg Traumatol 2013;23 Suppl 1:S93-100. [Crossref] [PubMed]
- Bilsky MH, Boland P, Lis E, et al. Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine (Phila Pa 1976) 2000;25:2240-9, discussion 2250. [Crossref] [PubMed]
- Xiong W, Xu Y, Fang Z, et al. Total En Bloc Spondylectomy for Lumbar Spinal Tumors by Paraspinal Approach. World Neurosurg 2018;120:28-35. [Crossref] [PubMed]
- Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg 2001;94:232-44. [PubMed]
- Sorteberg A. Balloon occlusion tests and therapeutic vessel occlusions revisited: when, when not, and how. AJNR Am J Neuroradiol 2014;35:862-5. [Crossref] [PubMed]
- Hsieh PC, Gallia GL, Sciubba DM, et al. En bloc excisions of chordomas in the cervical spine: review of five consecutive cases with more than 4-year follow-up. Spine (Phila Pa 1976) 2011;36:E1581-7. [Crossref] [PubMed]
- Mattei TA, Mendel E. En bloc resection of primary malignant bone tumors of the cervical spine. Acta Neurochir (Wien) 2014;156:2159-64. [Crossref] [PubMed]
- Zileli M, Kilincer C, Ersahin Y, et al. Primary tumors of the cervical spine: a retrospective review of 35 surgically managed cases. Spine J 2007;7:165-73. [Crossref] [PubMed]
- Yang X, Wu Z, Xiao J, et al. Chondrosarcomas of the cervical and cervicothoracic spine: surgical management and long-term clinical outcome. J Spinal Disord Tech 2012;25:1-9. [Crossref] [PubMed]
- Neo M, Asato R, Honda K, et al. Transmaxillary and transmandibular approach to a C1 chordoma. Spine (Phila Pa 1976) 2007;32:E236-9. [Crossref] [PubMed]
- Oppenlander ME, Maulucci CM, Ghobrial GM, et al. En bloc resection of upper thoracic chordoma via a combined simultaneous anterolateral thoracoscopic and posterior approach. Neurosurgery 2014;10 Suppl 3:380-6; discussion 386. [Crossref] [PubMed]
- Boriani S, Bandiera S, Colangeli S, et al. En bloc resection of primary tumors of the thoracic spine: indications, planning, morbidity. Neurol Res 2014;36:566-76. [Crossref] [PubMed]
- Sciubba DM, Gokaslan ZL, Black JH 3rd, et al. 5-Level spondylectomy for en bloc resection of thoracic chordoma: case report. Neurosurgery 2011;69:onsE248-55; discussion onsE255-6.
- Goomany A, Timothy J, Robson C, et al. En bloc resection of a thoracic chordoma is possible using minimally invasive anterior access: An 8-year follow-up. J Neurosci Rural Pract 2016;7:138-40. [Crossref] [PubMed]
- Mody GN, Bravo Iniguez C, Armstrong K, et al. Early Surgical Outcomes of En Bloc Resection Requiring Vertebrectomy for Malignancy Invading the Thoracic Spine. Ann Thorac Surg 2016;101:231-6; discussion 236-7. [Crossref] [PubMed]
- Cappuccio M, Gasbarrini A, Donthineni R, et al. Thoracoscopic assisted en bloc resection of a spine tumor. Eur Spine J 2011;20 Suppl 2:S202-5. [Crossref] [PubMed]
- Clarke MJ, Hsu W, Suk I, et al. Three-level en bloc spondylectomy for chordoma. Neurosurgery 2011;68:325-33; discussion 333. [PubMed]
- Kawahara N, Tomita K, Murakami H, et al. Total en bloc spondylectomy of the lower lumbar spine: a surgical techniques of combined posterior-anterior approach. Spine (Phila Pa 1976) 2011;36:74-82. [Crossref] [PubMed]
- Huth JF, Dawson EG, Eilber FR. Abdominosacral resection for malignant tumors of the sacrum. Am J Surg 1984;148:157-61. [Crossref] [PubMed]
- Localio SA, Francis KC, Rossano PG. Abdominosacral resection of sacrococcygeal chordoma. Ann Surg 1967;166:394-402. [Crossref] [PubMed]
- Tomita K, Tsuchiya H. Total sacrectomy and reconstruction for huge sacral tumors. Spine (Phila Pa 1976) 1990;15:1223-7. [Crossref] [PubMed]
- Clarke MJ, Dasenbrock H, Bydon A, et al. Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. Neurosurgery 2012;71:357-64; discussion 364. [Crossref] [PubMed]
- Bydon M, De la Garza-Ramos R, Bettegowda C, et al. En Bloc Resection of a Giant Cell Tumor in the Sacrum via a Posterior-Only Approach Without Nerve Root Sacrifice: Technical Case Report. Neurosurgery 2015;11 Suppl 3:E472-8. [Crossref] [PubMed]
- Clarke MJ, Zadnik PL, Groves ML, et al. En bloc hemisacrectomy and internal hemipelvectomy via the posterior approach. J Neurosurg Spine 2014;21:458-67. [Crossref] [PubMed]


