
Routine intensive monitoring but not routine intensive care unit-based management is necessary in video-assisted thoracoscopic surgery lobectomy for lung cancer
Introduction
The immediate postoperative care of patients that have undergone lobectomy is a critical aspect of patient recovery and can be challenging (1). Various cardiopulmonary complications are responsible for significant numbers of deaths and morbidities after lung cancer surgery (2,3). Recently, the advents of minimally invasive surgical techniques and video-assisted thoracic surgery (VATS) have greatly improved the early postoperative outcomes of lung cancer patients as compared with conventional thoracotomy (4-7).
Despite relatively low operative mortality rates, the majority of patients are still routinely monitored in intensive care units (ICUs) immediately after surgery in many centers (8,9). Even low risk patients have been routinely admitted to the ICU during the immediate postoperative period for monitoring purpose. However, in this era of minimally invasive surgery, this strategy has drawbacks such as delaying surgery schedules if an ICU bed is not available, causing problems such as cough deprivation, and delirium during the ICU stay, and increasing medical and economic burdens.
Globally, the needs of VATS lobectomy for lung cancer are increasing due to early detection and treatment, and extension of surgical indications. Therefore, the potential demand of ICU is anticipated to increase, and the routine ICU care may not only be clinically ineffective but result in the inefficient use of limited ICU resources and increase health care costs. Semi-ICU is considered as an alternative to ICU for postoperative patients (8). However, many hospitals do not have a semi-ICU because it requires specialized medical personnel and resources. The aim of this study was to compare the clinical benefits and cost-effectiveness of general ward (GW) care and routine ICU care during the immediate postoperative period in lung cancer patients after VATS lobectomy. We compared the postoperative outcomes including complications between two groups after propensity score matching. Furthermore, we evaluated the clinical factors associated with complications, and stratified patients according to the risk.
Methods
This study was based on a retrospective review of the medical records of 451 patients that underwent VATS lobectomy for lung cancer from June 1, 2012 to August 30, 2017. We excluded patients undergoing pneumonectomy or extended resection. The institutional review board of our center approved this study and waived the requirement for informed consent.
Lobectomy was considered when expected postoperative forced expiratory volume in one second (FEV1) was more than 40% without major hypoxemia (<60 mmHg) or hypercapnia (46 mmHg). Resection including mediastinal lymph node dissection was performed through VATS under general anesthesia with selective single lung ventilation in clinical stage I and II. Patients were allocated to an ICU group (immediate ICU transfer after surgery) or a GW group (immediate GW transfer after surgery), according to postoperative management strategies. In our center, selected patients were received care in the GW according to the availability of ICU. Patients who are older than 75 years, has a history of TIA and cerebral infarction without residuals (patients who has history of cerebrovascular accident with significant sequelae were excluded from surgery indication), and an expected FEV1 less than 60% were excluded from GW care. Most patients of ICU group stayed in the ICU for 24 hours after surgery. The duration of ICU stays were decided depending on patient’s conditions and severity after surgery. GW group was transferred from the recovery room to the GW after 1 hour, if their conditions were stable after surgery. Clinical data were collected for all patients from operative and anesthesia records, and nursing notes, and included age, sex, smoking status, tumor size, American Society of Anesthesiologists (ASA) grade, pathologic stage, operative time, preoperative pulmonary function test results, estimated blood loss and postoperative hospital stay. The comorbidities recorded included diabetes, hypertension, liver cirrhosis, chronic kidney disease, cerebrovascular disease, coronary artery disease, atrial fibrillation, heart failure, chronic obstructive pulmonary disease (COPD), and bronchiectasis. All postoperative complications were recorded including prolonged air leak (>5 days), transfusion on postoperative day 0, pneumonia, chylothorax, wound infection, reintubation, readmission to ICU, arrhythmia and mortality. Immediate complications were defined as occurring within 24 hours after surgery. Non-immediate complications were defined as occurring after 24 hours of surgery. Mortality was defined as all surgery related deaths that occurred within 1 month after surgery or during the hospitalization period. Hospital charges are presented as the sum of all costs incurred for all hospital stays in US dollars (exchange rate: 1,106.3 Korean won/$). To evaluate the safety and feasibility of GW care, the outcomes including complications, postoperative hospital stay, and hospital costs of both groups were compared after propensity score matching for variables such as age, sex, and smoking status. In addition, to decide the selection criteria for GW care following VATS lobectomy, clinical factors for complications were evaluated.
Statistical analysis
Statistical analysis was conducted using SPSS for Windows (ver. 21.0; IBM Corp., Armonk, NY, USA). Continuous variables are expressed as means ± standard deviation and were compared using the student’s t-test. Categorical values are expressed as percentages and were compared using the χ2 test or Fisher’s exact test, as appropriate. In this study, the clinical factors for total complications were evaluated because of low incidence of immediate complications. Univariate logistic regression analysis was performed to determine associations between baseline clinical characteristics and complication. Multivariate analysis was performed using the binary logistic regression model with backward stepwise method. Results are expressed as odds ratios (ORs) with 95% confidence interval (CI) and two-tailed P values of <0.05 were considered significant. To stratify the patients into groups according to their risk for complications, we used the variables remaining significant (P<0.05) in the multivariate analysis to construct a scoring system. We assessed how well the model can discriminate between patients with and without complications using a receiver operating characteristic (ROC) curve and the area under the curve (AUC). The AUC can be understood as the probability that a randomly chosen patient with complications will have a higher score than a randomly chosen patient without complications.
Results
Patient characteristics
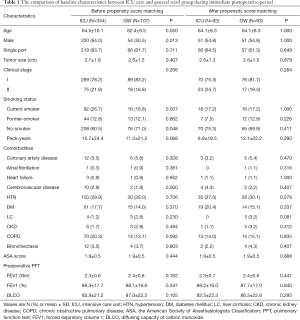
A total of 451 patients were included in the study. Thoracoscopic pulmonary resections were successfully performed in all patients. Three hundred forty four patients were in ICU group, and 107 patients were in GW group. The two groups differed in age, sex and smoking habit (Table 1). Mean ages in the ICU and GW groups were 64.5 and 62.4 years, respectively (P=0.050). The proportion of male was higher in the ICU (64.0% vs. 50.5%, P=0.013). The proportion of current smoker was significantly higher in the ICU (26.7% vs. 16.8%, P=0.037). There were no significant differences of clinical state, comorbidities, ASA, and preoperative PFT in the two groups. A total of 286 patients underwent single port VATS lobectomy and 165 patients multiport VATS lobectomy. Average duration of immediate postoperative ICU stay was 1.4 days in the ICU group.
Full table
Postoperative complications
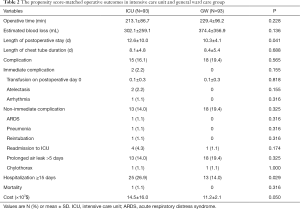
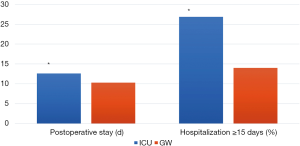
Immediate complication occurred in 2 patients (0.4%), which were atelectasis (N=2) and arrhythmia (N=1). However, non-immediate complication occurred in 85 patients (18.8%). There was no postoperative bleeding, and reoperation was not necessary. The type of late complication are as following; prolonged air leak (N=70, 15.5%), pneumonia (N=14, 3.1%), chylothorax (N=8, 1.8%), and ARDS (N=4, 0.9%). Sixteen patients were readmitted to the ICU, and the most common cause of ICU readmission was pneumonia requiring mechanical ventilation (N=12). The mean ICU readmission occurred 9.6±10.8 days after surgery. Mortality occurred in 1 patient; he died due to multi-organ failure related to pneumonia. There were no significant differences in the rates of immediate complication, non-immediate complication, and mortality between two groups after 1:1 propensity score matching (GW 93 vs. ICU 93) (Table 2). However, the mean postoperative stay (12.6±10.0 vs. 10.3±4.1, P=0.041) of the ICU group was significantly higher than the GW group (Figure 1). The hospital costs of the ICU group was higher than the GW group, but there was no statistical significance (×1,000, $, 14.5±16.0 vs. 11.2±2.1, P=0.050).
Full table
Risk factors of complication after VATS lobectomy
In univariate regression analysis, male (OR =0.51, 95% CI: 0.30–0.86, P=0.012), smoker (OR =3.66, 95% CI: 2.24–5.99, P<0.001), COPD (OR =5.62, 95% CI: 3.32–9.53, P<0.001), non-stage I (OR =1.97, 95% CI: 1.16–3.35, P=0.013), multiport (OR =3.71, 95% CI: 2.27–6.06, P<0.001), preoperative FEV1 <80 (OR =1.90, 95% CI: 1.15–3.12, P=0.012) and age ≥60 (OR =2.29, 95% CI: 1.24–4.24, P=0.008) were associated with complication after VATS lobectomy. Multivariate analysis including these factors showed that COPD (OR =3.00, 95% CI: 1.51–5.97, P=0.002), non-stage I (OR =2.54, 95% CI: 1.39–4.62, P=0.002), multi-port surgery (OR =3.75, 95% CI: 2.18–6.44, P<0.001) and age ≥60 (OR =2.12, 95% CI: 1.03–4.37, P=0.042) were independently associated with complication after VATS lobectomy (Table 3).

Full table
Scoring system for overall complications
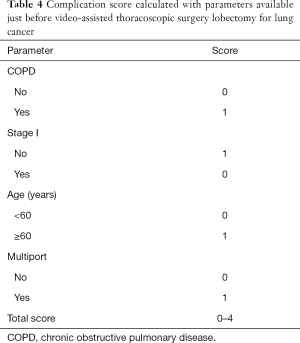
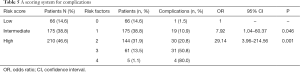
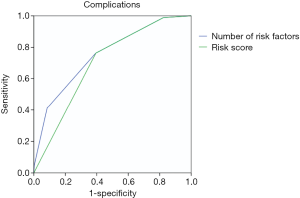
Despite the differences in the regression coefficients (β), for simplicity, 1 point was assigned for each of the risk factors (Table 4). The 3 risk groups were established; low risk (no risk factors, 14.6%), intermediate risk (1 risk factor, 38.8%), and high risk (2–4 risk factors, 46.6%, Table 5). Complication rates were highest in the high risk group (low vs. intermediate vs. high; 1.5% vs. 10.9% vs. 31.0%, P<0.001). The relative risk of complications in the intermediate and high risk groups compared with the low risk group was 7.92 (95% CI: 1.04–60.37, P=0.046), and 29.14 (95% CI: 3.96–214.56, P=0.001). The area under the ROC curve was 0.752 (0.695–0.809) for the logistic regression model and 0.702 (0.646–0.757) for the simplified score for complications (Figure S1).
Full table
Full table
Discussion
In this study, GW care did not increase the incidence of complications or mortality compared with ICU care, and monitoring of immediate postoperative management did not fail either. Immediate complication occurred in 0.4%, and a large proportion of patients that undergo VATS lobectomy do not need the routine intensive monitoring during the immediate postoperative period. Therefore, selective intensive monitoring for high risk groups may offer cost-saving and efficient use of ICU resources instead of customary ICU monitoring during immediate postoperative period.
Recent years have witnessed a trend toward minimally invasive surgery. Furthermore, the VATS approach has been further developed to reduce both the size and number of access incisions (6).Although this minimally invasive approach is intended to shorten postoperative recovery times, postoperative recovery protocols are still geared to address slow recovery after open thoracotomy, and current postoperative management remains the rate-limiting step during patient recovery. In this context, routine ICU use has been questioned, as indiscriminate, routine ICU use appears to be of little value in terms of predicting complications or management decision making. Thus, given increasing emphasis on cost effectiveness and limited ICU resources, this aspect of patient care requires critical evaluation (8). Several institutions use semi-ICU as an alternative to ICU, but this also has limitations because it requires specialized medical personnel and resources. Even in South Korea, which is not a low economic country, many hospitals do not have a semi-ICU. Recently, GW is being used as an alternative to ICU with advances in anesthesiology, surgery, and perioperative management. The main concern about the location of postoperative care is whether the GW is as safe as the ICU. However, this issue has scarcely been evaluated (9-11).
In this study, the safety and feasibility of GW care was comparable with that of ICU care after VATS lobectomy. The routine ICU admission did not significantly affect the overall postoperative complication rate. Immediate complication was always atelectasis, which were not fatal and were mostly treatable in the GW. However, non-immediate complications were mainly pneumonia required ICU readmission for mechanical ventilation (1 patient led to mortality). These results indicate that immediate postoperative ICU care does not prevent severe complications or deaths after VATS lobectomy. On the contrary, unnecessary ICU stay may lead to postoperative pneumonia following exposure to nosocomial infections or delayed early ambulation and rehabilitation (12). It has been reported that early mobilization due to adoption of a non-ICU based policy improves outcomes after surgery by preventing functional decline, reducing pain, enhancing well-being, and reducing hospital stays (13). In the present study, significant intergroup differences were between postoperative stays and hospital costs, which suggest routine ICU use may prolong recovery. Furthermore, by not utilizing expensive ICU resources, immediate postoperative monitoring in a GW could potentially improve overall treatment efficacy by efficiently providing ICU resources for more appropriate patients (14).
Therefore, it is necessary to establish criteria to judge whether or not ICU admission. Traditionally, there are several studies on the risk factors associated with postoperative complications (2,15-18). In this study, COPD, non-stage I, multiport surgery and age ≥60 were independent risk factors for complications. We developed a simple scoring system based on these risk factors to determine the standard of the location of the immediate postoperative care. In particular, the highest risk group had a 29.14-fold higher risk of complications than those of the lowest risk group. Therefore, selective intensive monitoring for these risk groups may offer cost-saving and efficient use of ICU resources instead of customary ICU monitoring during immediate postoperative period. This score could be helpful in the decision of the location of the immediate postoperative care, predominantly those with low risk groups, which could increase the treatment efficacy at lower cost.
The present study is inherently limited by its retrospective, single center design. Furthermore, this study cannot show the risk factors for immediate complication due to small case number who had immediate complication. However, it also has several advantages. This study performed with a homogenous group of patients, and all procedures, preoperative evaluations and postoperative care were conducted in the same surgical team with a homogenous manner. Furthermore, these patients analyzed after propensity score matching to reduce the effects of confounding factor.
Conclusions
This study shows it is possible to manage patients safely in a GW after VATS lobectomy for lung cancer. However, it should be noted that COPD, non-stage I cancer, multiple port VATS and age ≥60 have been shown to be independently associated with postoperative complications (19). Therefore, the patient without these risk factors can be managed safely in GW immediately after VATS lobectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of our center approved this study and waived the requirement for informed consent.
References
- Stéphan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70. [Crossref] [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Licker M, de Perrot M, Hohn L, et al. Perioperative mortality and major cardio-pulmonary complications after lung surgery for non-small cell carcinoma. Eur J Cardiothorac Surg 1999;15:314-9. [Crossref] [PubMed]
- Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by Video-Assisted Thoracic Surgery vs. Muscle-Sparing Thoracotomy for Stage I Lung Cancer: A Critical Evaluation of Short- and Long-Term Outcomes. J Am Coll Surg 2015;220:1044-53. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Chen D, Du M, Yang T. Uniportal video-assisted thoracoscopic lobectomy for lung cancer. J Thorac Dis 2016;8:1830-3. [Crossref] [PubMed]
- Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [Crossref] [PubMed]
- Murphy DJ, Lyu PF, Gregg SR, et al. Using Incentives to Improve Resource Utilization: A Quasi-Experimental Evaluation of an ICU Quality Improvement Program. Crit Care Med 2016;44:162-70. [Crossref] [PubMed]
- Melley DD, Thomson EM, Page SP, et al. Incidence, duration and causes of intensive care unit admission following pulmonary resection for malignancy. Intensive Care Med 2006;32:1419-22. [Crossref] [PubMed]
- Wyser C, Stulz P, Soler M, et al. Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 1999;159:1450-6. [Crossref] [PubMed]
- Schweizer A, Khatchatourian G, Hohn L, et al. Opening of a new postanesthesia care unit: impact on critical care utilization and complications following major vascular and thoracic surgery. J Clin Anesth 2002;14:486-93. [Crossref] [PubMed]
- Burgmann H, Hiesmayr JM, Savey A, et al. Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med 2010;36:1597-601. [Crossref] [PubMed]
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec 2012;26:87-94. [Crossref] [PubMed]
- Perez d'Empaire PA, Kajdacsy-Balla Amaral AC. Year in review 2011: Critical Care--Resource management. Crit Care 2012;16:244. [Crossref] [PubMed]
- Irie M, Nakanishi R, Yasuda M, et al. Risk factors for short-term outcomes after thoracoscopic lobectomy for lung cancer. Eur Respir J 2016;48:495-503. [Crossref] [PubMed]
- Lai Y, Su J, Wang M, et al. Classification and Risk-factor Analysis of Postoperative Cardio-pulmonary Complications after Lobectomy in Patients with Stage I Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2016;19:286-92. [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- McCall PJ, Macfie A, Kinsella J, et al. Critical care after lung resection: CALoR 1, a single-centre pilot study Anaesthesia 2015;70:1382-9. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. A propensity matched comparison of effects between video assisted thoracoscopic single-port, two-port and three-port pulmonary resection on lung cancer. J Thorac Dis 2016;8:1469-76. [Crossref] [PubMed]


