Norepinephrine, more than a vasopressor
In a recent study, Hamzaoui et al. (1) reported that early use of norepinephrine (NE) in patients with septic shock increased cardiac systolic function despite the increase in left ventricular afterload secondary to the rise in mean arterial pressure (MAP).
Background
NE is the alpha-adrenergic vasopressor of choice in septic shock (2) because of its ability to restore both arterial tone and the effective circulating volume. The latter would be the result of recruitment of unstressed volume from the venous capacitance vessels, with an increase in stressed volume, mean systemic filling pressure (Pmsf) and the driving pressure for venous return (Pvr) (3). In addition, by its beta-1 and alpha-1 adrenergic properties, NE also increases contractility of the myocardium (4). Nevertheless, an increase in left ventricular afterload and the resistance to venous return (Rvr) may ultimately decrease cardiac output (CO) (3).
NE and its effect on CO
Given the previous facts, the effect of NE on CO is highly variable and may depend on baseline cardiac preload status and on its inotropic effects. As an increase in preload also results in an increase in myocardial contraction force by the Starling mechanism (5), the real inotropic effect of NE is not easy to assess. In an early study by Goldberg et al. (6), the researchers showed an increase in right ventricular contractile force in thoracotomy patients, when infusing NE. In 1965 Cohn et al. observed that for a similar increase in MAP in a mixed group of patients with circulatory shock, the use of NE was associated with more pronounced increase in CO when compared to angiotensin, an agent lacking venoconstriction properties (7). In post-cardiac surgery patients, Maas et al. (3) found an increase in CO when increasing NE, associated with a decrease in stroke volume variation (SVV) from 14.4% to 11.9%, while patients that exhibited a decrease in CO were fluid unresponsive at baseline (SVV 9.1%). Hamzaoui et al. (1) showed a significant increase in LVEF and stroke volume, after adding or titrating NE to reach a MAP >65 mmHg in a subgroup of patients with septic shock and left ventricular ejection fraction (LVEF) <45%. In septic shock patients increasing the dose of NE reduces the effect of a passive leg raising on CO (8). Indeed, decreasing NE dose should lead to a decrease in Pmsf, Pvr and subsequently venous return and CO. This was confirmed in a recent study (9).
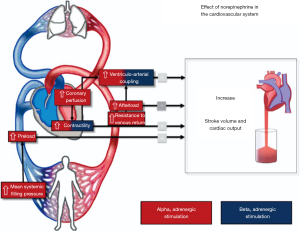
Likewise, titrating NE in fluid resistant hypotensive post cardiac surgery patients improved stroke volume and ventriculo-arterial (V-A) coupling (10). V-A coupling is another important determinant of cardiovascular performance. V-A coupling can be measured as the ratio of arterial elastance (Ea) to end-systolic ventricular elastance (Ees) (11). Ea is the expression of the total afterload imposed on the left ventricle and represents the complex association of different arterial properties including wall stiffness, compliance and outflow resistance; Ees is a useful, relatively load independent index of myocardial contractility (11,12) although it is affected by baseline myocardial characteristics as stiffness, compliance, fibrosis, synchrony, and remodeling (13). The majority of patients with septic shock exhibit significant V-A decoupling independent of the commonly seen reduced Ea (14). The assessment of cardiovascular function by evaluation of the Ea/Ees ratio can offer an adjunctive perspective for understanding the pathophysiology of altered hemodynamic profiles. NE may have mainly inotropic effects in case of LV dysfunction and mainly vasoconstrictive effects in case of arterial dysfunction. Correction of either of these variables results in improvement of ventriculo-arterial coupling (Figure 1).
Methodology and reliability to assess cardiac function with echocardiography in ICU patients
LVEF is a classical measurement of cardiac function, representing how much blood is being pumped out of the left ventricle. Robotham et al. (15). more than 25 years ago suggested that LVEF reflects the coupling between left ventricular contractility and afterload. In the same direction, Jardin et al. (16) described that patients with a normal LVEF had a significantly lower systemic vascular resistance (SVR) than patients with impaired LVEF. Thus, LVEF reflects the fact that vasoplegia unmasks systolic cardiac dysfunction. In addition, LVEF can improve by an increase in end-diastolic volume due to fluid resuscitation and by decreased afterload due to low systemic resistance. Likewise LVEF decreases as afterload increases, the differences being greatest at low preloads (15). Therefore, LVEF is the result of an interaction between preload, contractility and afterload, and its normality does not rule out myocardial depression (15).
Systolic function can be assessed by tissular Doppler imaging (TDI) through its systolic mitral annulus velocity (Sm), reflecting the peak velocity of shortening of the myocardial fibers oriented in the longitudinal direction. In an experimental study, A’roch et al. (17) found no change in Sm when venous return of the inferior vena cava was stopped (inflated balloon), but it increased with inotropic drugs. Likewise, Amà et al. (18) observed a significant increase in Sm after a volume challenge in cardiac surgery patients with conserved ejection fraction, and no changes in Sm after an increase in afterload with phenylephrine. Nonetheless, Oki et al. (19) showed a significant decrease of Sm when afterload was increased in healthy volunteers. Thus, TDI Sm appears being more sensible to increases in venous return, afterload and inotropic drugs than to decreases on venous return (17-19).
The complexity of right ventricle (RV) anatomy and geometry challenges the accurate assessment of right ventricular systolic function. TDI also has the potential to assess ventricular contractile function independent of the shape of the ventricle. The tricuspid lateral annular systolic velocity (Sa) can assess the longitudinal myocardial velocities as the tricuspid annulus descends toward the apex. Nonetheless, experimental models found that Sa is reduced by both, a decrease in preload and an increase in afterload (20), and that Sa increases with inotropes and fluid challenges (21). Thus, the magnitude of ejection phase myocardial velocities has been shown to be preload and afterload dependent (19). In the same direction, TAPSE (tricuspid annular plane systolic excursion), which represents longitudinal movement of the free wall of the RV base, correlates strongly with RV ejection fraction obtained by radionuclide angiography (22). Unfortunately, TAPSE also is limited by both preload and afterload dependency (23).
The ideal index of ventricular performance should be sensitive to contractile changes, independent of loading conditions, and easily obtained and reproducible (12). Through the analysis of the end-systole pressure volume relationship (ESPVR), the end systole elastance (Ees) is a relatively load independent parameter, combined with its adequate reflection of inotropic state (11). However, studies in animals and patients have shown that it cannot be relied on under all operating conditions because of load dependencies, despite being relatively load-independent over a specified range (12).
Conclusions
Several mechanisms contribute to preserve adequate cardiac function in patients with circulatory failure: appropriate preload, good contractility, and reduced afterload, as well as an optimal ventriculo-arterial coupling. In this setting, the study by Hamzaoui et al. (1) and earlier studies show that the use of NE, even early in the course of septic shock, has no significant deleterious effect on myocardial function even if MAP is significantly improved. Caution should however be taken when the patient is fluid unresponsive and thus already operating on the flat part of the Starling’s curve.
Acknowledgements
The authors are grateful with M.D. David Carpio Cordero for the figure design.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hamzaoui O, Jozwiak M, Geffriaud T, et al. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br J Anaesth 2018;120:517-24. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 2013;41:143-50. [Crossref] [PubMed]
- Skomedal T, Borthne K, Aass H, et al. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor-mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. J Pharmacol Exp Ther 1997;280:721-9. [PubMed]
- Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol 1914;48:357-79. [Crossref] [PubMed]
- Goldberg LI, Bloodwell RD, Braunwald E, et al. The direct effects of norepinephrine, epinephrine, and methoxamine on myocardial contractile force in man. Circulation 1960;22:1125-32. [Crossref] [PubMed]
- Cohn JN, Luria MH. Studies in Clinical Shock and Hypotension. Ii. Hemodynamic Effects of Norepinephrine and Angiotensin. J Clin Invest 1965;44:1494-504. [Crossref] [PubMed]
- Monnet X, Jabot J, Maizel J, et al. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011;39:689-94. [Crossref] [PubMed]
- Persichini R, Silva S, Teboul JL, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012;40:3146-53. [Crossref] [PubMed]
- Guinot PG, Longrois D, Kamel S, et al. Ventriculo-Arterial Coupling Analysis Predicts the Hemodynamic Response to Norepinephrine in Hypotensive Postoperative Patients: A Prospective Observational Study. Crit Care Med 2018;46:e17-25. [Crossref] [PubMed]
- Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983;245:H773-80. [PubMed]
- Kass DA, Maughan WL, Guo ZM, et al. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation 1987;76:1422-36. [Crossref] [PubMed]
- Guarracino F, Baldassarri R, Pinsky MR. Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 2013;17:213. [Crossref] [PubMed]
- Guarracino F, Ferro B, Morelli A, et al. Ventriculoarterial decoupling in human septic shock. Crit Care 2014;18:R80. [Crossref] [PubMed]
- Robotham JL, Takata M, Berman M, et al. Ejection fraction revisited. Anesthesiology 1991;74:172-83. [Crossref] [PubMed]
- Jardin F, Brun-Ney D, Auvert B, et al. Sepsis-related cardiogenic shock. Crit Care Med 1990;18:1055-60. [Crossref] [PubMed]
- A'Roch R, Gustafsson U, Johansson G, et al. Left ventricular strain and peak systolic velocity: responses to controlled changes in load and contractility, explored in a porcine model. Cardiovasc Ultrasound 2012;10:22. [Crossref] [PubMed]
- Amà R, Segers P, Roosens C, et al. The effects of load on systolic mitral annular velocity by tissue Doppler imaging. Anesth Analg 2004;99:332-8. table of contents. [PubMed]
- Oki T, Fukuda K, Tabata T, et al. Effect of an acute increase in afterload on left ventricular regional wall motion velocity in healthy subjects. J Am Soc Echocardiogr 1999;12:476-83. [Crossref] [PubMed]
- Vogel M, Schmidt MR, Kristiansen SB, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation 2002;105:1693-9. [Crossref] [PubMed]
- Hori Y, Kunihiro S, Hoshi F, et al. Comparison of the myocardial performance index derived by use of pulsed Doppler echocardiography and tissue Doppler imaging in dogs with volume overload. Am J Vet Res 2007;68:1177-82. [Crossref] [PubMed]
- Kaul S, Tei C, Hopkins JM, et al. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 1984;107:526-31. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]