
Quadriceps tendinopathy: a review—part 1: epidemiology and diagnosis
Introduction
Anterior knee pain is a very common problem seen in the outpatient clinics focused on musculoskeletal disorders, which has a broad differential, and may be often generalized by practitioners as chondromalacia patella and/or patellofemoral syndrome (1). However, quadriceps tendinopathy is an important cause of anterior knee pain, that is most commonly seen in athletes due to chronic degenerative tendon changes from repetitive loading, stress, and extension of the knee (2-5). It is often found co-existing with patellar tendinosis, which most commonly involves the tendon distally, but also the quadriceps muscle proximally. Historically, both entities have been labeled as Jumper’s knee due to the high prevalence seen in athletes, and both have often been treated in a similar manner due to their similarity (2,5-9). In many published reports, the name “patellar tendinopathy” is used to describe tendinopathy of the quadriceps tendon. More recently, an association of anterior knee pain and patellar tendinosis in community-based non-athletic patients, who have an increased body mass index (BMI) was found (10). These findings highlight the importance of surveillance of this entity as a cause of anterior knee pain in the non-athlete population.
Quadriceps tendinopathy is a clinical diagnosis characterized by activity-related anterior knee pain with localized tenderness at the superior border of the patella. Multiple authors have developed classification schemes which correlate persistence of symptoms with activity levels and have categorized them into early and late stages (5,11,12). The use of diagnostic imaging such as radiographs, magnetic resonance imaging (MRI), and various forms of ultrasound such as grey-scale, high resolution, color Doppler, and elastography have been studied extensively in athletes (13-18). It is not uncommon for asymptomatic athletes to have structural changes within tendons, however, symptomatic patients have consistently revealed morphologic changes of localized tendon thickening, hypoechoic areas, and increased vascularity (13,14). While conventional imaging modalities detect tendon structural changes, a strong link between structural changes and clinical symptoms does not exist (19,20). The need for future correlative imaging studies to assess structural changes might prove invaluable for the diagnosis and management of these disorders.
Historically, management of quadriceps tendinopathy has been based on the classification systems of Blazina, Roels, and Ferretti which correlated treatment based on the stage of symptoms. It is initially managed non-operatively. In the earlier stages of quadriceps tendinopathy, this approach has shown superior outcomes compared to surgical treatment, which is typically reserved for later stages and/or patients who have failed first-line non-operative measures (4,9,12,21,22). In addition, injections of platelet rich plasma (PRP) and sclerosing agents such as polidocanol have shown symptomatic relief in those who have failed first line conservative measures (23-26).
To the best of our knowledge, there is limited literature focusing directly on quadriceps tendinopathy, especially in the non-athlete population. In addition, there is a clear demand for both a gold standard in diagnostic imaging, as well as the need for a standard classification system for this and all tendon pathologies. Therefore, we performed a comprehensive literature review of these studies. This review consists of two parts. In the first part we review: (I) epidemiology and (II) diagnosis of quadriceps tendinopathy in the athlete as well as the general population. In the second part we discuss: (I) classification; (II) prognosis; and (III) treatment results.
Methods
A comprehensive literature search using PubMed, EBSCO Host, and SCOPUS was performed for this review of quadriceps tendinopathy. We searched studies from January 1977 to January 2017.
We used various combinations of the following terms to perform the search: [quadriceps tendinosis] [quadriceps tendonitis], [quadriceps tendinopathy], [patellar tendinosis], [patellar tendinopathy], and [jumper’s knee].
We reviewed 106 abstracts to determine reports that might be appropriate and identified 62 potentially relevant studies for further evaluation. We excluded 26 reports that were not in English or not relevant to the current topic. We excluded 7 studies performed on animal subjects. We then assessed the references of reports and found an additional 5. Therefore: 34 studies were included in the final review.
Results
Epidemiology
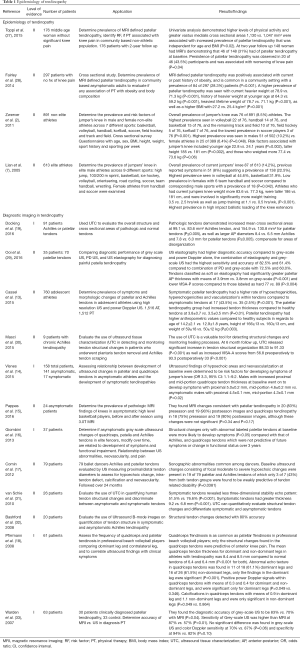
Multiple studies have demonstrated quadriceps tendinosis in patients due to high stress and repetitive loads on the extensor mechanism of the knee (Table 1). A retrospective study of 613 athletes by Lian et al. (7) examined the prevalence of jumper’s knee across 9 different sports.
Full table
Overall prevalence of jumper’s knee was seen in 14.2% athletes. In addition, 8% reported previous symptoms, suggesting a prevalence of current or prior symptoms in 22%. The highest prevalence was seen in sports associated with high impact ballistic loading of the knee extensors: volleyball (44.6%), basketball (31.9%), while there were no cases in cycling (P<0.001). Lowest prevalence was seen in combined team handball and soccer in both men (13.5%) and women (5.6%; P=0.042). Athletes who had current jumpers knee had a higher mean weight (83.6 vs. 77.3 kg; P<0.001), were taller (186 vs. 181 cm; P<0.001), and were involved in significantly more weight training (3.5 vs. 2.5 hrs/wk; P<0.001), as well as jump training (1.1 vs. 0.5 hrs/wk; P<0.001).
A cross sectional survey by Zwerver et al. (2) evaluated the prevalence and risk factors of jumper’s knee in 891 non-elite athletes across 7 different sports. Overall prevalence of jumper’s knee was 8.5%, with the highest prevalence seen in volleyball players at 14.4%, followed by handball 13.3%, basketball 11.8%, track and field 6.9%, field hockey 5.1%, korfball 4.8%, and soccer 2.5% (P=0.001). Higher prevalence’s were seen in males (10.2%) compared to females (6.4%; P=0.048). Risk factors associated with jumper’s knee included younger age (23 vs. 24 years; P=0.002), taller height (185 vs. 181 cm; P=0.002), and higher weight (77.4 vs. 73.6 kg; P=0.06).
A retrospective study of 176 non-athlete patients in a community setting at two-year follow up performed by Toppi et al. (27) examined the prevalence of patellar tendinopathy defined by MRI, risk factors, and association of patellar tendinopathy with pain. Overall prevalence of MRI-defined patellar tendinopathy was 30.1%. Univariate analysis demonstrated that higher levels of physical activity and greater vastus medialis muscle cross sectional areas (1,130 vs. 1,047 mm2) were associated with an increased prevalence of patellar tendinopathy that was independent of age and BMI (P=0.02). A total of 148 women had MRIs at two year follow up, of which 31% had patellar tendinopathy at baseline. Persistence of patellar tendinopathy was observed in 43.5% and was associated with worsening of knee pain (P=0.04). A study of 297 non-athlete asymptomatic patients of ages between 50 and 79 years by Fairley et al. (28) demonstrated that MRI-defined patellar tendinopathy was positively associated with a current or past history of obesity, and was common in a community setting with a prevalence of 28.3% (P<0.001). Higher prevalence’s of patellar tendinopathy was seen with taller heights (170 vs. 167 cm; P<0.001), current heavier weight at 78.9 vs. 71.3 kg (P<0.001), history of heavier weight at younger ages (18 to 21 years old) at 64.3 vs. 59.2 kg (P<0.001), heaviest lifetime weight of 78.7 vs. 71.1 kg (P<0.001), a higher BMI (27.2 vs. 25.4 kg/m2; P=0.001), as well as in males compared to females (54% vs. 46%; P<0.001). Furthermore, fat free mass and fat mass measurements were obtained in 78% of participants. A higher prevalence of patellar tendinopathy was seen in patients with a higher fat free mass (52 vs. 44 kg; P<0.001), however, there was no significant difference in fat mass between the two groups (28 vs. 26 kg; P=0.21).
In summary, quadriceps tendinopathy has been extensively studied in the athletic population. More recent studies reveal quadriceps tendinopathy does exist in non-athlete patients in a community setting, and there are associations linked to obesity, overall heavier weights, and increased height. There is a clear need for further studies to identify risk factors and prevalence of quadriceps tendinopathy in the non-athlete population.
Diagnostic imaging
The use of diagnostic imaging including radiographs, MRI’s and various types of ultrasound techniques, such as grey-scale, high resolution, color Doppler, and elastography, have been studied extensively (16,17). The use of radiographs may demonstrate degenerative changes such as patellar pole elongation from osteophyte formation, as well as tendon calcifications (11,34). Structural changes within tendons may often be found in asymptomatic patients, which pose a challenge when predicting who will possibly become symptomatic.
A prospective study of 158 student volleyball players (312 tendons) performed by Visnes et al. (14) evaluated the relationship between hypoechoic changes and neovascularization within the patellar and quadriceps tendons and knee pain. They found that 16% of the asymptomatic athletes went on to develop activity-related anterior knee pain. Ultrasound findings of hypoechoic areas and neovascularization at baseline were determined by multivariate logistic regression to be risk factors for developing symptoms [odds ratio (OR) 3.3, 95% confidence interval (CI): 1.1–9.2]. Compared to an asymptomatic group, symptomatic athletes had increased baseline ultrasound changes of hypoechoic areas (55% vs. 10%) and neovascularizations (48% vs. 4%). In the symptomatic group, increases from baseline hypoechoic areas (83 from 55%) and neovascularizations (74 from 48%) were observed at the time of diagnosis which persisted until the last examination. In addition, among asymptomatic males, for those who went on to develop symptoms, there was a larger mean baseline tendon thickness, when compared to those who remained asymptomatic (proximal 5.0 vs. 4.5 mm; P=0.02; mid-portion 4.6 vs. 4.3 mm; P=0.02). No changes in patellar tendon thickness were observed in athletes. Pappas et al. (15) used MRIs to evaluate structural changes of tendons before and after a season of basketball in 24 asymptomatic collegiate basketball players. A high prevalence of MRI changes was found in asymptomatic athletes; MRI changes consistent with patellar tendinopathy in 83% preseason and 90% postseason images and quadriceps tendinopathy in 75% preseason and 90% postseason images. However, these changes were not significant, and did not have a clinical correlation (P=0.34, P=0.17). These results highlight the need for further research on diagnostic imaging.
Pfirrman et al. (18) sonographically evaluated the patellar and quadriceps tendons of 61 professional volleyball players, of which quadriceps tendinosis was diagnosed in 34 (55.7%). They found that only thickening and structural changes found in the quadriceps tendons were predictive of anterior knee pain. Compared to the asymptomatic group, symptomatic athlete’s had ultrasound changes consisting of increased mean quadriceps muscle tendon thickness (8.4 vs. 6.4 mm; P<0.001), loss of fiber visibility and hypoechoic areas in 16 (61%) of tendons (P=0.001), as well as a mean positive power Doppler signals of 0.3 (P=0.049).
Studies have also compared the diagnostic accuracy of different imaging modalities. A prospective study of 35 volleyball players (70 tendons) by Ooi et al. (29) found that the diagnostic accuracy of grey-scale US, power Doppler and US elastography for diagnosing patellar tendinopathy to be 60%, 50% and 62%, with sensitivities/specificities of 72.5%/43.3%, 12.5%/100%, and 70%/53.3%. The combination of elastography and grey-scale US had higher sensitivity (82.5% vs. 72.5%) and accuracy (61.4% vs. 60.0%), when compared to the combination of power Doppler and grey-scale US. A similar study by Warden et al. (33) evaluated 33 patients who had clinically diagnosed patellar tendinopathy and determined that grey-scale and color Doppler ultrasound were more accurate than MRI in confirming clinically diagnosed patellar tendinopathy. Compared to MRI, grey-scale US had a higher diagnostic accuracy (83% vs. 70%; P=0.04), as well as a sensitivity (87% vs. 57% P=0.01).
A retrospective study of 1,512 patellar tendons and 1,516 Achilles tendons from 760 adolescent athletes performed by Cassel et al. (13) determined the prevalence of tendinopathy, symptoms, and intratendinous changes of tendons using high resolution and power Doppler US. Symptomatic patellar tendinopathy had a higher rate of hypoechogenicities, hyperechogenicities and neovascularization within tendons compared to asymptomatic tendons (43.5% vs. 2.5%; P<0.001), as well as increased mean tendon thickness compared to healthy tendons (3.8 vs. 3.5 mm; P=0.01). Compared to healthy subjects, the patellar tendinopathy group had higher mean age (14.2 vs. 12.9 years), higher mean height (166 vs. 160 cm), and were heavier with mean weight (59 vs. 50 kg; P≤0.003).
New imaging modalities
Conventional imaging modalities have greatly improved the accuracy and sensitivity of detecting pathologic tendon changes. However, major limitations still exist in regard to the lack of a standardized classification scheme that correlates treatment protocols, as well as imaging that can quantify structural and mechanical properties. The need for future correlative imaging work to assess structural changes might prove invaluable for the diagnosis and management of quadriceps tendinopathy.
More recently, ultrasound tissue characterization (UTC) was developed for veterinary use to quantify tendon integrity of horses, with results validated by equine tendon histology (35,36). It’s use in humans has been also reported in several studies (37-39). UTC utilizes images over the length of the tendon to quantified three-dimensional stability. A case-control study by van Schie et al. (31) evaluated the use of UTC for quantifying the tendon structure in twenty-six human Achilles tendons with midportion tendinopathy. Based on algorithms created from equine studies, UTC stability echo patterns were assigned 4 different echo types: (I) highly stable; (II) medium stable; (III) highly variable; and (IV) constant low intensity and variable distribution. Type I and II structural echoes were characterized as stable, whereas, types III and IV were characterized as having a lack of stability. Compared to symptomatic tendons, asymptomatic tendons revealed a higher percentage of type I and II stability echo patterns (76.6% vs. 51.5%; P<0.001), and lower unstable type III and IV patterns (23.4% vs. 48.4%; P<0.001). Symptomatic tendons also demonstrated a greater thickness compared to the asymptomatic tendons (9.2 vs. 6.8 mm; P<0.001). In the symptomatic group, 3 of the tendons had normal tendon structure, and 6 of the asymptomatic tendons had abnormal structures. The accuracy of UTC quantification of tendon structures was determined to be 83%. Furthermore, this was the first study to determine the inter-observer reliability of ultrasound imaging which was at 0.92–0.95. However, this technique is relatively new, not commonly used, and has not been studied extensively in humans.
Among the different imaging modalities, ultrasound is a safe, inexpensive, and accurate tool that may be used in diagnosing quadriceps tendinopathy and helping to guide options for symptomatic relief. Although, structural changes may be found in asymptomatic patients, consistent changes are found within the tendons of symptomatic patients. Various forms of US can be used for this purpose including grey-scale, high resolution, color Doppler, and elastography, and UTC. To date, there is no gold standard imaging or classification scheme to guide treatment of tendinopathy. Development of an imaging based tendinopathy classification scheme would prove invaluable for diagnosis, management, and prognosis of quadriceps tendinopathy.
Acknowledgements
None.
Footnote
Conflicts of Interest: M Chughtai: Cymedica; DJ Orthopaedics; Peerwell; Performance Dynamics Inc.; Refelection; Sage Products; Stryker. P Saluan: AAOS Now; Arthrex, Inc.; Equalizer, LLC; Middle Path Innovations, LLC; Pediatric Orthopaedic Society of North America.MA Mont: AAOS, Cymedica, DJ Orthopaedics, Johnson & Johnson, Journal of Arthroplasty, Journal of Knee Surgery, Microport, National Institutes of Health (NIAMS & NICHD), Ongoing Care Solutions, Orthopedics, Orthosensor, Pacira, Peerwell, Performance Dynamics Inc., Sage, Stryker: IP royalties, Surgical Technologies International, Kolon TissueGene. J Genin: Ferring Pharmaceuticals. The other authors have no conflicts of interest to declare.
References
- Thomeé R, Augustsson J, Karlsson J. Patellofemoral pain syndrome: a review of current issues. Sports Med 1999;28:245-62. [Crossref] [PubMed]
- Zwerver J, Bredeweg SW, van den Akker-Scheek I. Prevalence of Jumper’s Knee Among Nonelite Athletes From Different Sports: A Cross-Sectional Survey. Am J Sports Med 2011;39:1984-8. [Crossref] [PubMed]
- Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med 2005;33:561-7. [Crossref] [PubMed]
- Ferretti A, Puddu G, Mariani PP, et al. The natural history of jumper’s knee. Patellar or quadriceps tendonitis. Int Orthop 1985;8:239-42. [Crossref] [PubMed]
- Blazina ME, Kerlan RK, Jobe FW, et al. Jumper’s knee. Orthop Clin North Am 1973;4:665-78. [PubMed]
- Ferretti A, Mariani PP, Neri M. The natural history of jumper’s knee. Int Orthop 1985;8:239-42. [Crossref] [PubMed]
- Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med 2005;33:561-7. [Crossref] [PubMed]
- Ferretti A. Epidemiology of jumper’s knee. Sports Med 1986;3:289-95. [Crossref] [PubMed]
- Martens M, Wouters P, Burssens A, et al. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand 1982;53:445-50. [Crossref] [PubMed]
- Toppi J, Fairley J, Cicuttini FM, et al. Factors associated with magnetic resonance imaging defined patellar tendinopathy in community-based middle-aged women: a prospective cohort study. BMC Musculoskelet Disord 2015;16:184. [Crossref] [PubMed]
- Roels J, Martens M, Mulier JC, et al. Patellar tendinitis (jumper’s knee). Am J Sports Med 6:362-8. [Crossref] [PubMed]
- Ferretti A, Conteduca F, Camerucci E, et al. Patellar tendinosis: a follow-up study of surgical treatment. J Bone Joint Surg Am 2002;84-A:2179-85. [Crossref] [PubMed]
- Cassel M, Baur H, Hirschmüller A, et al. Prevalence of Achilles and patellar tendinopathy and their association to intratendinous changes in adolescent athletes. Scand J Med Sci Sports 2015;25:e310-8. [Crossref] [PubMed]
- Visnes H, Tegnander A, Bahr R. Ultrasound characteristics of the patellar and quadriceps tendons among young elite athletes. Scand J Med Sci Sports 2015;25:205-15. [Crossref] [PubMed]
- Pappas GP, Vogelsong MA, Staroswiecki E, et al. Magnetic Resonance Imaging of Asymptomatic Knees in Collegiate Basketball Players: The Effect of One Season of Play. Clin J Sport Med 2016;26:483-9. [Crossref] [PubMed]
- Giombini A, Dragoni S, Di Cesare A, et al. Asymptomatic Achilles, patellar, and quadriceps tendinopathy: A longitudinal clinical and ultrasonographic study in elite fencers. Scand J Med Sci Sports 2013;23:311-6. [Crossref] [PubMed]
- Comin J, Cook JL, Malliaras P, et al. The prevalence and clinical significance of sonographic tendon abnormalities in asymptomatic ballet dancers: a 24-month longitudinal study. Br J Sports Med 2013;47:89-92. [Crossref] [PubMed]
- Pfirrmann CW, Jost B, Pirkl C, et al. Quadriceps tendinosis and patellar tendinosis in professional beach volleyball players: Sonographic findings in correlation with clinical symptoms. Eur Radiol 2008;18:1703-9. [Crossref] [PubMed]
- Cook JL, Rio E, Purdam CR, et al. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research?. Br J Sports Med 2016;50:1187-91. [Crossref] [PubMed]
- Rio E, Moseley L, Purdam C, et al. The pain of tendinopathy: physiological or pathophysiological?. Sports Med 2014;44:9-23. [Crossref] [PubMed]
- Cucurulo T, Louis ML, Thaunat M, et al. Surgical treatment of patellar tendinopathy in athletes. A retrospective multicentric study. Orthop Traumatol Surg Res 2009;95:S78-84. [Crossref] [PubMed]
- Santander J, Zarba E, Iraporda H, et al. Can arthroscopically assisted treatment of chronic patellar tendinopathy reduce pain and restore function? Clin Orthop Relat Res 2012;470:993-7. [Crossref] [PubMed]
- Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med 2014;42:610-8. [Crossref] [PubMed]
- Dallaudière B, Pesquer L, Meyer P, et al. Intratendinous injection of platelet-rich plasma under US guidance to treat tendinopathy: a long-term pilot study. J Vasc Interv Radiol 2014;25:717-23. [Crossref] [PubMed]
- Gosens T, Den Oudsten BL, Fievez E, et al. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: A prospective cohort study and the influence of previous treatments. Int Orthop 2012;36:1941-6. [Crossref] [PubMed]
- Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop 2010;34:909-15. [Crossref] [PubMed]
- Toppi J, Fairley J, Cicuttini FM, et al. Factors associated with magnetic resonance imaging defined patellar tendinopathy in community-based middle-aged women: a prospective cohort study. BMC Musculoskelet Disord 2015;16:184. [Crossref] [PubMed]
- Fairley J, Toppi J, Cicuttini FM, et al. Association between obesity and magnetic resonance imaging defined patellar tendinopathy in community-based adults: a cross-sectional study. BMC Musculoskelet Disord 2014;15:266. [Crossref] [PubMed]
- Ooi CC, Richards PJ, Maffulli N, et al. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J Sci Med Sport 2016;19:373-8. [Crossref] [PubMed]
- Masci L, Spang C, van Schie HT, et al. Achilles tendinopathy-do plantaris tendon removal and Achilles tendon scraping improve tendon structure? A prospective study using ultrasound tissue characterisation. BMJ open Sport Exerc Med 2015;1:e000005. [Crossref] [PubMed]
- van Schie HT, de Vos RJ, de Jonge S, et al. Ultrasonographic tissue characterisation of human Achilles tendons: quantification of tendon structure through a novel non-invasive approach. Br J Sports Med 2010;44:1153-9. [Crossref] [PubMed]
- Bashford GR, Tomsen N, Arya S, et al. Tendinopathy Discrimination by Use of Spatial Frequency Parameters in Ultrasound B-Mode Images. IEEE Trans Med Imaging 2008;27:608-15. [Crossref] [PubMed]
- Warden SJ, Kiss ZS, Malara FA, et al. Comparative Accuracy of Magnetic Resonance Imaging and Ultrasonography in Confirming Clinically Diagnosed Patellar Tendinopathy. Am J Sports Med 2007;35:427-36. [Crossref] [PubMed]
- Greenspan A, Norman A, Tchang FK. “Tooth” sign in patellar degenerative disease. J Bone Joint Surg Am 1977;59:483-5. [Crossref] [PubMed]
- van Schie HT, Bakker EM, Jonker AM, et al. Computerized ultrasonographic tissue characterization of equine superficial digital flexor tendons by means of stability quantification of echo patterns in contiguous transverse ultrasonographic images. Am J Vet Res 2003;64:366-75. [Crossref] [PubMed]
- van Schie HT, Bakker EM, Cherdchutham W, et al. Monitoring of the repair process of surgically created lesions in equine superficial digital flexor tendons by use of computerized ultrasonography. Am J Vet Res 2009;70:37-48. [Crossref] [PubMed]
- Docking SI, Rosengarten SD, Cook J. Achilles tendon structure improves on UTC imaging over a 5-month pre-season in elite Australian football players. Scand J Med Sci Sports 2016;26:557-63. [Crossref] [PubMed]
- Docking SI, Cook J. Pathological tendons maintain sufficient aligned fibrillar structure on ultrasound tissue characterization (UTC). Scand J Med Sci Sports 2016;26:675-83. [Crossref] [PubMed]
- Aström M, Gentz CF, Nilsson P, et al. Imaging in chronic achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol 1996;25:615-20. [Crossref] [PubMed]


