Systematic review on the utility of magnetic resonance imaging for operative management and follow-up for primary sarcoma—lessons from extremity sarcomas
Background
Primary tumors of the vertebral column tumors are a rare clinical entity with a reported incidence of 0.14–0.19 persons per 100,000 population annually, increasing steadily with patient age (1). These lesions can be divided into benign and malignant varieties, of which the latter account for 50–67% of clinical cases (1). While benign lesions are often best initially managed with conservative therapies addressed at the patient’s symptomatology (2), malignancies (including chordoma, chondrosarcoma, Ewing’s sarcoma, osteosarcoma, and plasmacytoma) are often treated through surgical management (3).
Pre-operative evaluation of patients being considered for surgical management can be divided into pathology, tumor grading, and tumor location/operative characteristics. Pathology consists of CT-guided needle biopsy (4), which confirms the malignant nature of the tumor and indicates the amenability of the tumor to non-surgical management. In the absence of mechanical instability, plasmacytoma is best managed through a combination of radiation and CyBorD (cyclophosphamide-bortezomib- dexamethasone) chemotherapy and Ewing sarcoma benefits from multimodal management with surgery and chemoradiation (5); all others are treated principally with surgical resection when feasible. Grading—the histological description of tumor differentiation and potential aggressiveness—is then performed using the system presented by Enneking in 1980 and later refined in 1986 (6,7). Lastly, the Weinstein-Boriani-Biagini system is applied to localize the tumor within the spine, identifying the approach, feasibility of en bloc resection, and potential need for instrumentation (4,8).
Originally developed for appendicular lesions, the Enneking system has since become a staple of pre-operative planning for primary vertebral malignancies (9). It prescribes proper surgical margins for lesions based upon histologic features. For primary vertebral column malignancies, the goal is negative margins, as this typically decreases local recurrence and may improve mortality (10,11). Consequently, being able to pre-operatively identify dissection planes that will produce negative margins is paramount. Currently magnetic resonance imaging (MRI) with and without contrast is the gold standard due to its high-resolution. Additionally, routine MRI is used to assess residual disease and as a surveillance tool for local recurrence. Despite the accepted superiority of MR, little to no primary literature exists evaluating the diagnostic utility of MR for pre-operative or post-operative evaluation of spinal malignancies. Several studies have been done in patients with primary osseous and soft tissue sarcomas of the periphery however. Here we review this literature as a means of describing the likely accuracy of MR for pre- and post-operative evaluation of primary vertebral malignancy.
Literature search
We performed a systematic review of the existing literature on October 21, 2018 using the PubMed and EMBASE databases and the search strings identified in Table 1. Articles were identified based upon their ability to address one of the following questions:

Full table
- How accurate is pre-operative MR for assessing tumor margins in primary bone malignancies?
- To what degree can pre-operative MR imaging be used to guide osteotomy formation for en bloc resection of primary bone tumors?
- How accurate is post-operative MR imaging for the diagnosis of residual disease following resection of soft tissue sarcoma?
- How accurate is post-operative MR imaging for the diagnosis of disease recurrence following resection of soft tissue sarcoma?
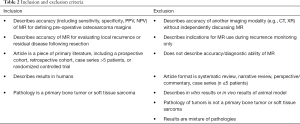
The inclusion and exclusion criteria for the study are outlined in Table 2. Only studies involving humans with English full-text translations in peer-reviewed journals were considered for inclusion; conference proceedings and poster presentations without accompanying manuscripts were excluded. Title and abstract screening was performed concurrently by two authors (Z Pennington and AK Ahmed) with discrepancies being resolved by a third author (EM Westbroek). Articles meeting criteria for full-text review underwent the same scrutiny and those meeting inclusion criteria had data abstracted by a single author (Z Pennington) and confirmed by a second author (AK Ahmed).
Full table
Results
Search results
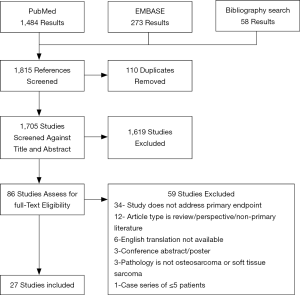
Our queries yielded 1,705 unique results, of which 1,619 were excluded as irrelevant based upon title and abstract. Full texts of the remaining 86 studies were then reviewed for inclusion, of which 27 studies met the inclusion criteria (Figure 1). The most common reason for exclusion was that the article failed to address one of the four questions used to focus the review. Overall, the results were too heterogenous to perform a meta-analysis.
How accurate is pre-operative MR for assessing tumor margins in primary bone malignancies?
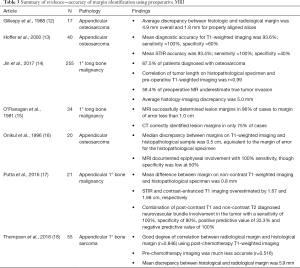
Seven articles published results describing the accuracy of MR for identifying pre-operative margins (Table 3) (12-18). Three evaluated the accuracy with which MR was able to assess margins for appendicular osteosarcoma, where the remaining four assessed accuracy across several different primary osseous malignancies of the appendicular skeleton. Of those studies reporting test accuracy characteristics, sensitivity of pre-operative MR for tumor margins was 100% and specificity varied between 50% and 60% (13,16) with an overall diagnostic accuracy of 93.6–96% (13,15). Thompson et al. (18) and Jin et al. (14) presented two large series of appendicular bone sarcoma with a combined 310 patients. Both groups correlated pre-operative margins on T1-weighted imaging with post-operative histological margins. Thompson and colleagues reported a correlation of 0.846 between the two measures with a mean difference of 5.9 mm. Jin et al. reported even greater correlation (r=0.99) with an average discrepancy of 5.0 mm, though the direction of this discrepancy led to underestimation of tumor invasion in 58.4% of cases. The high level of accuracy reported by these newer studies is similar to that reported by the previous work of O’Flanagan (15), Onikul (16) and Gillespy (12), who all reported mean discrepancies between MR and histological findings of less than 1.0 cm. Gillespy et al. noted that the discrepancy was reduced roughly three-fold for properly aligned slices, with a mean discrepancy of 1.8 mm (12). Putta et al. noted a similarly small discrepancy in their evaluation of 21 patients, finding a mean difference between histological margin and radiological margin of 0.8 mm using non-contrast T1-weighted imaging (17). They found that employing contrast imaging and using STIR sequence imaging both substantially increased the error in radiological margins, overestimating true tumor size by 1.68 and 1.67 cm, respectively. However, the authors did note that the use of fat-saturated, post-contrast-T1-weighted imaging was useful for identifying involvement of the neurovascular bundle. Combined post-contrast T1 imaging and T2 imaging was able to identify neurovascular bundle involvement with a sensitivity of 100% and specificity of 90%. Aggregated, the studies found non-contrast T1-weighted MR to be an accurate means of determining pre-operative margins for primary osseous malignancies.
Full table
To what degree can pre-operative MR imaging be used to guide osteotomy formation for en bloc resection of primary bone tumors?
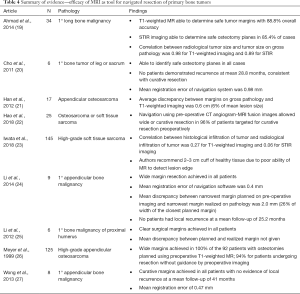
Nine articles published results describing the utility and accuracy with which MR-guidance can facilitate en bloc resection with negative margins (19-27) (Table 4). Eight of the articles examined osseous malignancies, three of which focused on appendicular osteosarcoma, while two studies described the results for soft tissue sarcoma, with Hao et al., 2018 (22) reporting a mixed cohort. The articles examining exclusively osseous malignancy reported a total of 205 patients. Overall, the proportion of patients achieving clean margins was high, ranging from 88.8% to 100% of patients. Two studies—Ahmad et al. (19) and Iwata et al. (23)—compared the accuracy of different imaging sequences for the guidance of resection. Both studies found T1-weighted imaging to mediate better guidance. Ahmad and colleagues reported a stronger correlation of radiological lesion size on T1-weighted imaging with size on gross pathology (r=0.98) as compared to STIR (r=0.89) for primary osseous malignancies. The authors reported that this discrepancy may stem from the visualized peritumoral edema seen on STIR volumes. Iwata et al. found that for soft tissue sarcoma, the overall correlation of tumor invasion on imaging and gross histology was much weaker than the correlation of size reported for bony tumor. However, post-contrast fat-saturated T1-weighted imaging was significantly better than STIR at predicting size (r=0.27 for T1 vs. r=0.06 for STIR).
Full table
Four studies reported local recurrence rates following navigated resection of primary osseous malignancies (20,24,25,27). Of the 23 patients included, all had wide margin excision, and none experienced local recurrence at a mean of 31.6 months. Though all groups described the importance of including a healthy tissue cuff of at least 2 cm, the overall accuracy of the navigation system was quite high. Across all included studies, the mean registration error between image and actual anatomy was 0.4–0.98 mm. Additionally, both Han et al. and Li et al. reported the mean discrepancy between osteotomy as planned and osteotomy as executed (21,24). The mean discrepancy across both studies was 4 mm or less, with Li et al. reporting a discrepancy of only 2.0 mm, or roughly 26% the width of their closest margin (24). In all studies, the authors concluded that MR was a necessary component for successful navigated resection, including in Hao et al., who employed MR/CT fusion images (22).
How accurate is post-operative MR imaging for the diagnosis of residual disease following resection of soft tissue sarcoma?
Our search yielded 5 studies discussing the utility of MR for diagnosing residual disease (Table 5) (28-32). Combined, the five studies included 299 patients, all having undergone prior resection of soft tissue sarcoma. Not controlling for differences in MR sequence(s) employed, the mean sensitivity of MR imaging for residual disease was found to be 71.7% (range: 60–86.7%) and specificity was found to be 79.3% (range: 57.9–93%) at a mean of 43.5 days post-resection (30-32). Three studies—those of Davies, Kaste, and Patkar—examined the relative diagnostic utility of contrast and non-contrast images and found no significant difference in diagnostic accuracy between contrast and non-contrast image sets (28,30,31). One study—that of Puhaindran et al. —compared the diagnostic utility of MR for residual disease as a function of the size of the residual lesion (32). Blocking tumors into gross residual disease and microscopic residual disease, the authors found that the overall diagnostic utility of MR was significantly improved for cases with gross residual disease, with a sensitivity of 89%, as compared to 60% for the overall cohort. None of the studies quantitatively assessed the ability of MR to distinguish residual disease from surgical bed edema or post-radiation changes, though several of the authors reported that these both may lower the discriminative ability of MR.

Full table
How accurate is post-operative MR imaging for the diagnosis of disease recurrence following resection of soft tissue sarcoma?
Six articles published results discussing the utility of MR for demonstrating recurrence following radical resection of soft-tissue sarcoma (Table 6) (33-38). Combined, the studies included 341 patients with soft tissue sarcoma. Including all MR sequences, the overall sensitivity of MR for local recurrence was found to be 87.9% (range: 83–100%) and the overall specificity was 85.9% (range: 55.6–100%). Of the included studies, three compared the diagnostic utility of MR to other imaging modalities, with Erfanian et al. comparing MR to PET/MR, Park et al. comparing MR to PET/CT, and Reuther and Mutschler comparing MR to CT (36-38). Erfanian et al. found that the addition of 18F-FDG PET to the follow-up regimen increased diagnostic accuracy from 80.7% to 89.5%, with a noticeable increase in sensitivity from 80.0% to 95.0%, albeit at the cost of a slight decrease in specificity (82.4% vs. 76.5%) (36). Park et al. by contrast found no significant difference between the diagnostic accuracy of MR (93.9%) and PET-CT (95.5%) for elucidating local recurrence, though the authors noted that MR had the advantages of: (I) no additional irradiation and (II) delineation of local anatomy for surgical planning in the case of recurrence (37). Lastly, Reuther and Mutschler found that MR was noticeably superior to CT for the evaluation of recurrence (92.6% vs. 85%), again with the benefit of not exposing patients to additional irradiation (38).
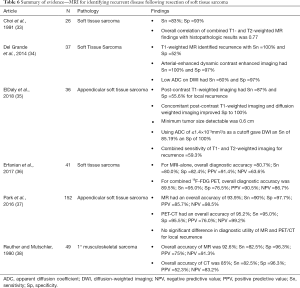
Full table
Of the six studies, two evaluated the use of diffusion weighted imaging (DWI) as part of the imaging protocol. Del Grande et al. and ElDaly et al. both found that the addition of DWI may increase the specificity of MR for local recurrence, with Del Grande et al. reporting a specificity of 97% with DWI and ElDaly et al. reporting a specificity of 100% with DWI (34,35). Additionally, ElDaly et al. reported that using an apparent diffusion coefficient cutoff of ≤1.4×10-3 mm2/s, overall sensitivity for recurrence was 85.19% and overall specificity was 100% with a minimum detectable tumor size of 0.6 cm in diameter (34). No quantitative evaluations between contrast and non-contrast MR were reported though several authors commented on contrast imaging being a valuable intervention from distinguishing tumor from edema or post-radiation changes.
Discussion
Learning points from the existing literature
As high-grade malignancies, osteosarcoma and soft tissue sarcoma of the extremities are currently best treated by en bloc resection with negative margins (39). Soft tissue sarcomas can frequently abut or involve key neurovascular structures. Needless sacrifice of these structures again hinders quality of life, but careless exclusion may prevent local control and therefore negatively impact long-term prognosis. It can be seen then that identifying a means of accurately assessing tumor margin preoperatively and guiding cuts along planned margins could confer significant benefit to existing surgical technologies.
The literature reviewed above supports the notion that magnetic resonance imaging is invaluable to the musculoskeletal surgical oncologist. Overall, the accuracy of MR for pre-operative margin evaluation appears to be exquisite, especially for osseous malignancy, with Jin et al. reporting a correlation between histopathologic and radiologic tumor size of 99%. Because of this accuracy, pre-operative MRI is routinely used during surgical planning for determination of osteotomy placement (40). Additionally, several groups have employed MR or MR-CT fusion images (22) for osteotomy formation under intraoperative computer-assisted navigation (20,24,25,27). Of the four groups here that employed intraoperative navigation, wide excision was achieved in 100% of cases. Local control was achieved in all patients, demonstrating that MR-navigated surgery may aid in the performance of Enneking-appropriate interventions. Furthermore, the studies presented indicate that MR accurately demonstrates residual disease, especially in cases of macroscopic disease, and is highly effective at demonstrating disease recurrence, though the addition of 18F-FDG PET may improve diagnostic sensitivity, as well as allow for detection of distant metastases (36).
Though the evidence supports the value of MR, many questions remain to be answered. Three of note are: (I) Which MR sequence should be used for preoperatively planning? (II) What follow-up regimen should be employed to look for local recurrence and residual disease? and (III) Does earlier detection of residual disease improve patient outcomes?
Years of experience have demonstrated that no single MR sequence is best for the evaluation of musculoskeletal sarcoma, and by extension, no single MR will be best for the evaluation of primary tumors of the vertebral column (41). Rather, imaging for primary musculoskeletal sarcoma should include a minimum of two MR sequences—at least one T1-weighted or anatomic scan and at least one T2-weighted scan to evaluate soft tissue margins (42-44). T1-weighted imaging gives the best definition of bone marrow invasion (13,15,16) and accordingly will provide the best evidence for guiding osteotomy cuts (15,19,23). By contrast, T2 or spin-spin sequences are highly responsive to free water protons, which are classically enriched in the pseudocapsule produced by atrophy of tumor-adjacent soft tissues (45). Usage of fat-saturated T2 sequences (46) or short tau inversion recovery (STIR) additionally increase the conspicuity of the soft tissue component by attenuating the signal of the normally T2-hyperintense adipose tissue (46,47). It must be noted though that STIR sequences also boost the signal produced by peritumoral edema, which is seen in nearly 70% of musculoskeletal malignancy (48,49), and therefore may give a falsely increased estimate of lesion size (17,50-52). Additionally, this edema is common to both benign and malignant lesions, reducing the prognostic utility of scans aimed at highlighting it (53). However, STIR and T2-weighted sequences have added utility in vertebral column malignancy in that they provide the best means of evaluating neural compression (41). In the case of sizeable lesions, STIR is more sensitive for assessment of the soft-tissue mass than are fat-suppressed sequences, as suppression is often heterogeneous across the large field of view (41).
The usage of contrast-enhanced sequences is advocated by many authors, as gadolinium contrast agents have the ability to distinguish viable tumor from both necrotic tumor (41,47) and peritumoral edema (54). Post-contrast, fat-suppressed T1 weighted sequences also enhance visualization of associated soft-tissue masses, as the mass enhances relative to the suppressed soft tissue (46) Additionally, some evidence suggests that time to peak of the post-contrast T1-weighted signal can distinguish benign from malignant lesions in ≈80% of cases (47). However, contrast administration is not without risk; between 20 and 330 people of every 100,000 experience immediate hypersensitivity reactions to contrast administration and between 0.7 and 0.97 of every million doses of contrast are lethal (55-57). A recent editorial in JAMA even highlights the potential long-term health dangers associated with retained gadolinium (58).
Currently no universal guidelines exist surrounding monitoring for residual disease or recurrence following excision of primary bony malignancies due to the heterogenous clinical courses of the distinct pathologies and relatively low-quality evidence upon which current paradigms are based. Many providers recommend annual MR imaging of the primary site, which is consistent with the most recent recommendations made by the American Association of Orthopaedic Surgeons. However, other organizations, such as the British National Health Service officially only recommend routine chest X-ray to evaluate for pulmonary metastatic disease. Avoiding questions of health resource distribution, the proper follow-up regimen should be dictated by the accuracy of the diagnostic methods and the degree to which they may alter clinical management. The evidence presented here suggests that routine MR is capable of diagnosing residual disease and local recurrence with an overall accuracy of 77–94% (28,36-38). Given that: (I) many patients with curative resection die from metastatic disease, and (II) local recurrence has only inconsistently been linked to distant metastases (59,60), it has been questioned as to whether routine imaging for local recurrence leads to changes in patient care. Several studies, including those of Kasalak, Richardson, Cheney, Watts, George, and Rothermundt have evaluated the ability of routine MR to mediate early detection of recurrent musculoskeletal sarcoma (61-65). Kasalak, Rothermundt, and Cheney all reported that radiological recurrence was more often than not accompanied or preceded by clinical/symptomatic recurrence, undermining the value of serial follow-up (62,63,66). By contrast, Richardson et al. found that ¼ to ½ of soft tissue sarcoma patients will present with radiological recurrence first and Watts et al. found that 70% of musculoskeletal sarcoma patients present with radiological recurrence prior to symptomatic recurrence (61,64). George et al. reported an intermediate result, finding that routine MR imaging of the surgical bed could lead to earlier detection of recurrence in 49% of patients (65). Additionally, they reported that of these patients, 33% had an alteration in their treatment regimen as a result. Alternatively expressed, serial MR for local recurrence may lead to a change in management of as many as 1 in every 6 patients.
Application to vertebral column malignancy
Like sarcoma of the periphery, primary osseous malignancy of the vertebral column is best treated with en bloc resection. Achieving negative margins in this context is often times far more difficult however, as the close proximity of the spinal cord and exiting nerve roots leave little room for error. Accurately identifying tumor margins preoperatively is therefore paramount.
Given the similarity of treatment goals for tumors of the axial and appendicular skeleton, it is logical that advances in one field may be potentially implemented in the other. As described in this paper, pre-operative magnetic resonance imaging outlines tumor margins with a high degree of accuracy and can be effectively used intraoperatively to guide osteotomy cuts. This holds true in the spine as well, with the understanding that surgeons will be constantly working close to tumor margins given the confines of the spine and spinal cord.
Additionally, the ability of intraoperative image guidance to achieve negative margins in the appendicular literature must be couched by the fact that margins of 2–3 cm are commonplace (23) and planned margins of 5 cm are not entirely uncommon (14). Even in the context of a radiological margin that poorly represents the gross pathological margin—1 cm or more—the planned cuff of healthy tissue is likely to include all local disease, giving the appearance of a fail-proof technology. Such large margins may be unreasonable for vertebral body malignancy, and therefore to conclude the perfect translatability of this technology, it is necessary to demonstrate that curative resection can be consistently achieved with a much smaller margin for error. Sparse evidence exists to suggest that imaging accuracy is sufficient to mediate en bloc resection. At present, only 4 cases have been reported that describe the accuracy of using pre-operative MRI for intraoperative navigation and en bloc resection of primary vertebral body malignancy. In three of the four cases, negative margins were reported and in none of the cases were local recurrence or permanent deficit noted (67-69).
Assuming that the results of these select case series are generalizable, intraoperative navigation appears to be a viable tool for guiding Enneking-appropriate resection of vertebral body malignancy. The last question that must be answered then is whether MR is an effective means of: (I) looking for residual disease, and (II) monitoring for local recurrence. Based upon the literature from peripheral soft tissue sarcomas, the answer appears to be in the affirmative, but the quality of evidence is too low to make a definitive conclusion. Additionally, the literature examined—that of soft tissue sarcoma—does not consider the efficacy of MR for monitoring of a surgical bed adjacent to ferromagnetic hardware as is the case following vertebral column resection. To this end, it is likely that although routine (6–12 mo) multi-sequence MR imaging of the surgical bed may aid in evaluation for recurrence, local metallic artifact precludes its exclusive use. Instead, it may be necessary to use an adjuvant diagnostic imaging modality that is immune to local metallic artifact, such as 18F-FDG PET, which demonstrably increases diagnostic accuracy for recurrence in the sarcoma literature (36). PET follow-up is not without its own issues however, as the intervention is extremely costly and patients are exposed to additional radiation. It is unlikely that in the current cost-conscious medical system this intervention will be supported without high quality evidence to support its use. Consequently, the need remains for a high-quality means of diagnosing early recurrent disease.
Potential pitfalls
One issue not addressed in the included studies is the presence of micro-skip metastases—small tumor foci that are not observable on current pre-operative imaging modalities. A recent study by Takeyama et al. examining patients with pathologically-confirmed chordoma reported that these “micro skip” metastases may be found in over 40% of patients (70). Though 95% of lesions existed less than one centimeter from the lesion border, in two cases these “micro skip” metastases were found nearly 2 cm from the lesion border. Takeyama et al. additionally reported that the presence of “micro skip” metastases was related with significantly lower overall survival, local recurrence-free survival, and metastasis-free survival. As these lesions are segregated from the gross tumor border and invisible to conventional imaging, even accurate osteotomy planes (as determined by preoperative imaging) may fail to include them. Though this has not been explicitly investigated, work by researchers at the Massachusetts General Hospital has demonstrated the use of adjuvant or neoadjuvant radiation significantly reduces local recurrence-free survival (71). Interestingly, use of neoadjuvant or adjuvant radiation was the only predictor of increased local recurrence-free survival on multivariable analysis; R0 resection did not produce any difference in local recurrence-free survival. This suggests, that as observed in the Takeyama cohort, occult “micro skip” metastases may exist outside the tumor boundaries, comprising a sort of “neoplastic penumbra”. This penumbra is missed with en bloc resection alone, yet is doubtlessly included in the radiation field, explaining the superior overall and progression-free survivals in these patients (72). In part, these results may undermine the emphasis on achieving precise osteotomies and en bloc resection; however, it should be noted that radiation is not without its own risks, including catastrophic mechanical failure (73), tumor dedifferentiation, and induction of high-grade sarcoma (74). The latter are substantially more difficult to treat. Consequently, surgeons and patients, alike, must weigh the potential costs and benefits of adjuvant radiation, namely improved local control versus higher complication rates. Improved imaging that allows the identification of “micro skip” metastases as well as navigated-osteotomies aimed at including at least 5 mm of healthy tissue may help to alter this discussion by relegating radiation to only those patients with extremely high-risk lesions or evidence of positive margins. On the other hand, as most of the patients in the chordoma series above were treated with R0 resection, there exists the possibility that even patients with histologically-clean resections may benefit from adjuvant radiotherapy (71). These results have not been expanded to other primary malignancies though and therefore may not be generalizable to all primary vertebral column malignancies.
Conclusions
Using the extant soft tissue sarcoma and appendicular osteosarcoma literature as a learning ground for spine, it appears as if surgical margins prescribed by pre-operative magnetic resonance imaging are accurate assessments of true pathological margins. Consequently, pre-operative imaging, notably T1-weighted volumes, can be used to guide intraoperative maneuvers for the achievement of curative margins. By contrast, the extant literature on the accuracy of MR for evaluating recurrent disease undermines its utility and is largely reserved to the soft tissue sarcoma literature. The latter is not directly translatable to primary vertebral column malignancies that are reconstructed with metal instrumentation and thus have significant artifact that precludes high resolution looks at the soft tissue post-operatively. Consequently, though useful for pre-operative planning and potentially for intraoperative guidance, MR imaging may not be an effective means of evaluating local recurrence following en bloc resection in patients receiving concomitant instrumentation.
Acknowledgments
None.
Footnote
Conflicts of Interest: ML Goodwin: Consultant for ROM3, Augmedics; DM Sciubba: Consultant for Orthofix, Globus, K2M, Medtronic, Stryker, Baxter. Other authors have no conflicts of interest to declare.
References
- Sohn S, Kim J, Chung CK, et al. A Nation-Wide Epidemiological Study of Newly Diagnosed Primary Spine Tumor in the Adult Korean Population, 2009-2011. J Korean Neurosurg Soc 2017;60:195-204. [Crossref] [PubMed]
- Ropper AE, Cahill KS, Hanna JW, et al. Primary vertebral tumors: a review of epidemiologic, histological, and imaging findings, Part I: benign tumors. Neurosurgery 2011;69:1171-80. [Crossref] [PubMed]
- Ropper AE, Cahill KS, Hanna JW, et al. Primary Vertebral Tumors: A Review of Epidemiologic, Histological, and Imaging Findings, Part II: Locally Aggressive and Malignant Tumors. Neurosurgery 2012;70:211-9. [Crossref] [PubMed]
- Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine 1997;22:1036-44. [Crossref] [PubMed]
- Sciubba DM, Okuno SH, Dekutoski MB, et al. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine 2009;34:S58. [Crossref] [PubMed]
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980.106-20. [PubMed]
- Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986.9-24. [PubMed]
- Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine 1996;21:1927-31. [Crossref] [PubMed]
- Chan P, Boriani S, Fourney DR, et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine 2009;34:384-91. [Crossref] [PubMed]
- Bakker SH, Jacobs WCH, Pondaag W, et al. Chordoma: a systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur Spine J 2018;27:3043-58. [Crossref] [PubMed]
- Lador R, Bandiera S, Gasbarrini A, et al. Treatment of Spinal Tumors in a High Volume Center has Direct Impact on Local Recurrence, Morbidity, and Mortality. Clin Spine Surg 2017;30:E1074. [Crossref] [PubMed]
- Gillespy T, Manfrini M, Ruggieri P, et al. Staging of intraosseous extent of osteosarcoma: correlation of preoperative CT and MR imaging with pathologic macroslides. Radiology 1988;167:765-7. [Crossref] [PubMed]
- Hoffer FA, Nikanorov AY, Reddick WE, et al. Accuracy of MR imaging for detecting epiphyseal extension of osteosarcoma. Pediatr Radiol 2000;30:289-98. [Crossref] [PubMed]
- Jin T, Deng Z, Liu W, et al. Magnetic Resonance Imaging for the Assessment of Long Bone Tumors. Chin Med J 2017;130:2547-50. [Crossref] [PubMed]
- O'Flanagan SJ, Stack JP, McGee HM, et al. Imaging of intramedullary tumour spread in osteosarcoma. A comparison of techniques. J Bone Joint Surg Br 1991;73:998-1001. [Crossref] [PubMed]
- Onikul E, Fletcher BD, Parham DM, et al. Accuracy of MR imaging for estimating intraosseous extent of osteosarcoma. AJR Am J Roentgenol 1996;167:1211-5. [Crossref] [PubMed]
- Putta T, Gibikote S, Madhuri V, et al. Accuracy of Various MRI Sequences in Determining the Tumour Margin in Musculoskeletal Tumours. Pol J Radiol 2016;81:540-8. [PubMed]
- Thompson MJ, Shapton JC, Punt SE, et al. MRI Identification of the Osseous Extent of Pediatric Bone Sarcomas. Clin Orthop Relat Res 2018;476:559-64. [Crossref] [PubMed]
- Ahmad S, Stevenson J, Mangham C, et al. Accuracy of magnetic resonance imaging in planning the osseous resection margins of bony tumours in the proximal femur: based on coronal T1-weighted versus STIR images. Skeletal Radiol 2014;43:1679-86. [Crossref] [PubMed]
- Cho HS, Park I, Jeon I, et al. Direct application of MR images to computer-assisted bone tumor surgery. J Orthop Sci 2011;16:190-5. [Crossref] [PubMed]
- Han G, Wang Y, Bi W, et al. Magnetic resonance imaging is appropriate for determining the osteotomy plane for appendicular osteosarcoma after neoadjuvant chemotherapy. Med Oncol 2012;29:1347-53. [Crossref] [PubMed]
- Hao Y, Yang C, He J. The accurate surgical margin before surgery for malignant musculoskeletal tumors: a retrospective study. Am J Transl Res 2018;10:2324-34. [PubMed]
- Iwata S, Araki A, Funatsu H, et al. Optimal surgical margin for infiltrative soft tissue sarcomas: Assessing the efficacy of excising beyond the infiltration. J Surg Oncol 2018;118:525-31. [PubMed]
- Li J, Wang Z, Guo Z, et al. Precise resection and biological reconstruction under navigation guidance for young patients with juxta-articular bone sarcoma in lower extremity: preliminary report. J Pediatr Orthop 2014;34:101-8. [Crossref] [PubMed]
- Li J, Wang Z, Guo Z, et al. Precise resection and biological reconstruction for patients with bone sarcomas in the proximal humerus. J Reconstr Microsurg 2012;28:419-25. [PubMed]
- Meyer MS, Spanier SS, Moser M, et al. Evaluating marrow margins for resection of osteosarcoma. A modern approach. Clin Orthop Relat Res 1999.170-5. [PubMed]
- Wong KC, Kumta SM. Joint-preserving tumor resection and reconstruction using image-guided computer navigation. Clin Orthop Relat Res 2013;471:762-73. [Crossref] [PubMed]
- Davies AM, Mehr A, Parsonage S, et al. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol 2004;14:506-13. [Crossref] [PubMed]
- Gingrich AA, Elias A, Michael Lee C, et al. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J Surg Res 2017;208:26-32. [Crossref] [PubMed]
- Kaste SC, Hill A, Conley L, et al. Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop Relat Res 2002.204-11. [Crossref] [PubMed]
- Patkar S, Gulia A, Juvekar S, et al. Does magnetic resonance imaging accurately predict residual disease after unplanned excision of soft-tissue sarcomas? Indian J Cancer 2016;53:408-11. [PubMed]
- Puhaindran ME, Pratt J, Manoso MW, et al. Predictive value of magnetic resonance imaging in determining presence of residual disease after marginal excision of unsuspected soft tissue sarcomas of the hand. J Hand Surg Am 2010;35:1479-84. [Crossref] [PubMed]
- Choi H, Varma DG, Fornage BD, et al. Soft-tissue sarcoma: MR imaging vs sonography for detection of local recurrence after surgery. AJR Am J Roentgenol 1991;157:353-8. [Crossref] [PubMed]
- Del Grande F, Subhawong T, Weber K, et al. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology 2014;271:499-511. [Crossref] [PubMed]
- ElDaly MM, Moustafa AFI, Abdel-Meguid SMS, et al. Can MRI diffusion-weighted imaging identify postoperative residual/recurrent soft-tissue sarcomas? Indian J Radiol Imaging 2018;28:70-7. [Crossref] [PubMed]
- Erfanian Y, Grueneisen J, Kirchner J, et al. Integrated 18F-FDG PET/MRI compared to MRI alone for identification of local recurrences of soft tissue sarcomas: a comparison trial. Eur J Nucl Med Mol Imaging 2017;44:1823-31. [Crossref] [PubMed]
- Park SY, Chung HW, Chae SY, et al. Comparison of MRI and PET-CT in detecting the loco-regional recurrence of soft tissue sarcomas during surveillance. Skeletal Radiol 2016;45:1375-84. [Crossref] [PubMed]
- Reuther G, Mutschler W. Detection of local recurrent disease in musculoskeletal tumors: magnetic resonance imaging versus computed tomography. Skeletal Radiol 1990;19:85-90. [Crossref] [PubMed]
- Lamplot JD, Denduluri S, Qin J, et al. The Current and Future Therapies for Human Osteosarcoma. Curr Cancer Ther Rev 2013;9:55-77. [PubMed]
- Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg 2009;17:515-27. [Crossref] [PubMed]
- Rodallec MH, Feydy A, Larousserie F, et al. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics 2008;28:1019-41. [Crossref] [PubMed]
- Gebauer GP, Farjoodi P, Sciubba DM, et al. Magnetic resonance imaging of spine tumors: classification, differential diagnosis, and spectrum of disease. J Bone Joint Surg Am 2008;90:146-62. [Crossref] [PubMed]
- Silva FD, Pinheiro L, Cristofano C, et al. Magnetic Resonance Imaging in Pediatric Bone Tumors. Curr Radiol Rep 2014;2:77. [Crossref]
- Vanel D. MRI of bone metastases: the choice of the sequence. Cancer Imaging 2004;4:30-5. [Crossref] [PubMed]
- Bahig H, Roberge D, Bosch W, et al. Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 2013;86:298-303. [Crossref] [PubMed]
- Manaster BJ. Soft-tissue masses: optimal imaging protocol and reporting. AJR Am J Roentgenol 2013;201:505-14. [Crossref] [PubMed]
- Nascimento D, Suchard G, Hatem M, et al. The role of magnetic resonance imaging in the evaluation of bone tumours and tumour-like lesions. Insights Imaging 2014;5:419-40. [Crossref] [PubMed]
- Golfieri R, Baddeley H, Pringle JS, et al. Primary bone tumors. MR morphologic appearance correlated with pathologic examinations. Acta Radiol 1991;32:290-8. [Crossref] [PubMed]
- Hanna SL, Fletcher BD, Parham DM, et al. Muscle edema in musculoskeletal tumors: MR imaging characteristics and clinical significance. J Magn Reson Imaging 1991;1:441-9. [Crossref] [PubMed]
- Golfieri R, Baddeley H, Pringle JS, et al. The role of the STIR sequence in magnetic resonance imaging examination of bone tumours. Br J Radiol 1990;63:251-6. [Crossref] [PubMed]
- Tokuda O, Hayashi N, Matsunaga N. MRI of bone tumors: Fast STIR imaging as a substitute for T1-weighted contrast-enhanced fat-suppressed spin-echo imaging. J Magn Reson Imaging 2004;19:475-81. [Crossref] [PubMed]
- Tokuda O, Harada Y, Matsunaga N. MRI of soft-tissue tumors: fast STIR sequence as substitute for T1-weighted fat-suppressed contrast-enhanced spin-echo sequence. AJR Am J Roentgenol 2009;193:1607-14. [Crossref] [PubMed]
- Alyas F, James SL, Davies AM, et al. The role of MR imaging in the diagnostic characterisation of appendicular bone tumours and tumour-like conditions. Eur Radiol 2007;17:2675-86. [Crossref] [PubMed]
- Kroon HM, Bloem JL, Holscher HC, et al. MR imaging of edema accompanying benign and malignant bone tumors. Skeletal Radiol 1994;23:261-9. [Crossref] [PubMed]
- Prince MR, Zhang H, Zou Z, et al. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011;196:W138-43. [Crossref] [PubMed]
- Behzadi AH, Zhao Y, Farooq Z, et al. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology 2018;286:471-82. [Crossref] [PubMed]
- Jung JW, Kang H, Kim M, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012;264:414-22. [Crossref] [PubMed]
- Levine D, McDonald RJ, Kressel HY. Gadolinium Retention After Contrast-Enhanced MRI. JAMA 2018;320:1853-4. [Crossref] [PubMed]
- Koscielny S, Tubiana M. The link between local recurrence and distant metastases in human breast cancer. Int J Radiat Oncol Biol Phys 1999;43:11-24. [Crossref] [PubMed]
- Vicini FA, Kestin L, Huang R, et al. Does local recurrence affect the rate of distant metastases and survival in patients with early-stage breast carcinoma treated with breast-conserving therapy? Cancer 2003;97:910-9. [Crossref] [PubMed]
- Richardson K, Potter M, Damron TA. Image intensive soft tissue sarcoma surveillance uncovers pathology earlier than patient complaints but with frequent initially indeterminate lesions. J Surg Oncol 2016;113:818-22. [Crossref] [PubMed]
- Cheney MD, Giraud C, Goldberg SI, et al. MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol 2014;109:593-6. [Crossref] [PubMed]
- Rothermundt C, Whelan JS, Dileo P, et al. What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 2014;110:2420-6. [Crossref] [PubMed]
- Watts AC, Teoh K, Evans T, et al. MRI surveillance after resection for primary musculoskeletal sarcoma. J Bone Joint Surg Br 2008;90:484-7. [Crossref] [PubMed]
- George A, Grimer RJ. J James SL. Could Routine Magnetic Resonance Imaging Detect Local Recurrence of Musculoskeletal Sarcomas Earlier? A Cost-effectiveness Study. Indian J Orthop 2018;52:81-6. [Crossref] [PubMed]
- Kasalak Ö, Dammann A, Adams HJA, et al. Surveillance MRI for the detection of locally recurrent Ewing sarcoma seems futile. Skeletal Radiol 2018;47:1517-22. [Crossref] [PubMed]
- Al Eissa S, Al-Habib AF, Jahangiri FR. Computer-Assisted Navigation During an Anterior-Posterior En Bloc Resection of a Sacral Tumor. Cureus 2015;7:e373. [PubMed]
- Fujibayashi S, Neo M, Takemoto M, et al. Computer-assisted spinal osteotomy: a technical note and report of four cases. Spine 2010;35:E895. [Crossref] [PubMed]
- Guppy KH, Chakrabarti I, Banerjee A. The use of intraoperative navigation for complex upper cervical spine surgery. Neurosurg Focus 2014;36:E5. [Crossref] [PubMed]
- Akiyama T, Ogura K, Gokita T, et al. Analysis of the Infiltrative Features of Chordoma: The Relationship Between Micro-Skip Metastasis and Postoperative Outcomes. Ann Surg Oncol 2018;25:912-9. [Crossref] [PubMed]
- van Wulfften Palthe ODR, Tromp I, Ferreira A, et al. Sacral Chordoma: A Clinical Review Of 101 Cases With 30-Year Experience In A Single Institution. Spine J 2019;19:869-79. [Crossref] [PubMed]
- Kabolizadeh P, Chen Y, Liebsch N, et al. Updated Outcome and Analysis of Tumor Response in Mobile Spine and Sacral Chordoma Treated With Definitive High-Dose Photon/Proton Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;97:254-62. [Crossref] [PubMed]
- Osler P, Bredella MA, Hess KA, et al. Sacral Insufficiency Fractures are Common After High-dose Radiation for Sacral Chordomas Treated With or Without Surgery. Clin Orthop Relat Res 2016;474:766-72. [Crossref] [PubMed]
- Kato S, Gasbarrini A, Ghermandi R, et al. Spinal chordomas dedifferentiated to osteosarcoma: a report of two cases and a literature review. Eur Spine J 2016;25 Suppl 1:251-6. [Crossref] [PubMed]