
Management of presacral schwannomas—a 10-year multi-institutional series
Introduction
Schwannomas represent a relatively unique clinical entity among primary tumors of the spinal cord and vertebral column. As predominately intradural, extramedullary tumors (1-3), schwannomas are dissimilar to both vertebral column tumors and intramedullary lesions; they seldom destabilize the bony elements and precipitate symptoms of myelopathy in roughly 10% of cases (4,5). Additionally, most are clinically benign (6) and have no documented impact on patient mortality. Consequently, the main surgical indication for the majority of schwannomas is radicular pain. Despite this, a subset of schwannomas—those arising from the anterior sacral roots—may present with unique symptomatology.
Presacral/sacral schwannomas are quite rare, representing only 0.3–3.2% of all schwannomas (7) and 0.4–15% of all retrorectal tumors (8,9). They may initially present with pelvic or low back pain, incontinence, urinary urgency, and constipation (10). Lesions commonly remain asymptomatic for years, gradually displacing the pelvic viscera until mass effect becomes great enough to produce clinical features (10). Consequently, they may be quite large at time of detection (11). Here we present a series of 7 patients who underwent surgery for resection of giant presacral schwannoma (Table 1).
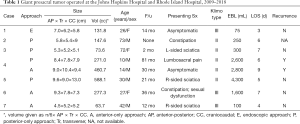
Full table
Case presentation
Case 1
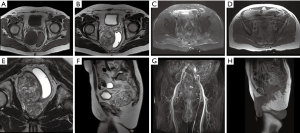
An asymptomatic 22-year-old woman presented for operative management of a large presacral mass identified on prior exploratory laparoscopy done to work-up abnormal uterine bleeding (Figure 1A,B,C,D,E,F). The patient was neurologically intact and serial magnetic resonance imaging (MRI) had demonstrated only minimal tumor growth since first identification (≈5%). The patient desired surgical intervention and underwent a robot-assisted anterior approach with resection of the presacral mass using the Da Vinci machine (Intuitive Surgical). The tumor was resected en bloc and the patient had an uneventful recovery, being discharged on post-operative day 3. She remained asymptomatic at 14-month follow-up (Figure 1G,H,L).

Case 2
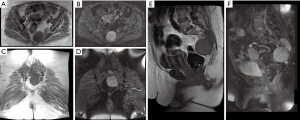
A 73-year-old man presented with a 2-month history of constipation and outside imaging demonstrating a large, homogenous presacral mass abutting the rectum, pelvic girdle, and right seminal vesicle (Figure 2A,B,C,D). The patient was otherwise asymptomatic. The patient did have a prior history of nephrectomy for renal cell carcinoma and so a posterior approach was offered. A two-level sacral laminectomy with distal sacral amputation was performed and the mass was delivered en bloc. Aside from minor voiding difficulty for the first 5 days post-operatively (presumably secondary to those roots feeding the presacral plexus), the patient had an uneventful recovery and demonstrated no residual disease on post-operative MRI (Figure 2E,F,G,H).
Case 3
A 72-year-old female with a recent stroke and residual left hemiparesis (arm greater than leg) presented with left-sided sciatic pain, progressive over 5 years, worst in the seated position. Outside imaging (Figure 3) had demonstrated a large presacral mass involving the left S2/3 and S3/4 foramina, consistent with presacral schwannoma. On exam, the patient demonstrated diffuse pain-associated weakness in the left lower extremity. Given the patient’s history of multiple prior abdominal surgeries, a posterior approach was offered. Gross total resection was achieved by internal debulking of the lesion followed by capsular dissection; this enabled preservation of the adjacent nerve roots. The patient had an uncomplicated recovery and was discharged home on POD 7. At 2-month follow-up, the patient endorsed significant improvement of her pre-operative pain and denied any new neurological deficit.
Case 4
A 10-year-old male presented with an 8-month history of progressive lumbosacral back pain exacerbated by exercise. The patient had a remote history of coccyx trauma and pain was initially attributed to this prior injury. After failure of conservative management, a pelvic MRI was obtained, documenting a large presacral mass involving the S2 and S3 foramina (Figure 4A,B,C,D,E,F). A posterior approach was pursued due to the intracanalicular portion of the lesion. Piecemeal dissection was performed; only subtotal resection was achieved due to the large size of the lesion (Figure 4G,H,I,J,K,L,M). The patient had an uneventful recovery without evidence of new neurological deficit.
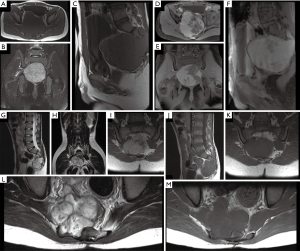
Given the residual disease, the tumor was monitored for growth on serial MRI. At 49 months follow-up, the mass had regrown to its pre-resection dimensions (now 9.0 cm × 10.4 cm × 9.4 cm) with radiographic evidence of significant mass effect on the rectum and colon (Figure 5A,B,C,D). Consequently, surgery was offered to prevent bowel dysfunction. Given that: (I) a posterior approach had previously been employed, and (II) the goal of surgery was to decompress the abdominopelvic viscera, a midline anterior approach was adopted. Greater than 95% of the mass was resected and aside from mild nausea the first 2 days, the patient had an uneventful recovery. Imaging at 5 months demonstrated residual tumor, though there was significant relief of mass effect on the rectum and bladder. At 30-month post-operative imaging, the mass remains largely unchanged with only minimal mass effect on the abdominopelvic viscera (Figure 5E,F,G,H,I,J).
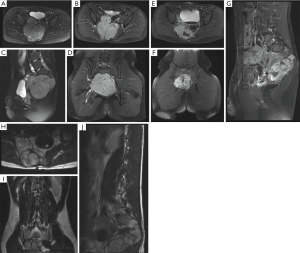
Case 5
A 30-year-old male presented with a 1 year history of progressive right-sided sciatic-type pain in the setting of a large presacral mass. His pain worsened with prolonged sitting and was unresponsive to over-the-counter analgesics. The patient additionally endorsed paresthesia in the right perineal region without alterations in gastrointestinal or sexual functions. He was neurologically nonfocal on exam, though review of his images were suggestive of some intradural component at S1 (Figure 6A,B,C,D). A hemisacrectomy with L3-pelvis instrumented fusion and resection of the presacral mass was recommended, as the tumor exhibited significant erosion of the right hemisacrum. At last follow-up, the patient remained neurologically intact with no signs of recurrence. He had some residual right-sided sciatic pain, which was attributed to chronic deformation of the nerve preoperatively (Figure 6E,F).
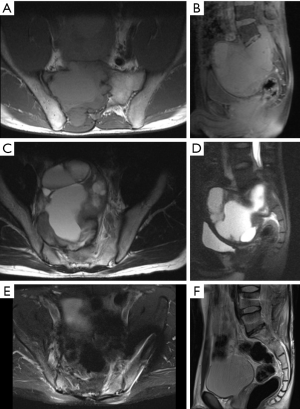
Case 6
A 27-year-old patient with documented history of neurofibromatosis type 2 presented with a large presacral mass compressing the rectum and vagina, causing significant constipation and vaginal obstruction. The patient denied significant pain symptoms and was neurologically intact on exam. The patient underwent a transabdominal approach with gross total resection, using serial internal debulking with an ultrasonic aspirator followed by capsular dissection. The patient awoke neurologically intact and had an uneventful inpatient stay. At 36-month follow-up, the resection site remained free of recurrence, though several new lesions were noted in the right sacrum and left sciatic notch consistent with the expected disease course of NF2. The patient remained neurologically intact with complete remission of preoperative symptoms.
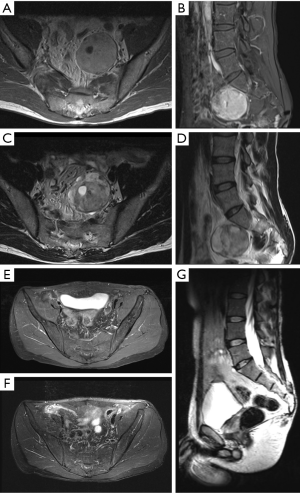
Case 7
A 42-year-old male presented with right lower extremity pain and numbness. The patient had been diagnosed with a presacral mass 10 years prior to presentation yet had foregone treatment due to only minimally bothersome symptoms at the time of diagnosis. On physical examination, the patient had full strength in all extremities, and normal rectal tone. Imaging demonstrated a 4.5 cm × 5.2 cm × 5.2 cm left-sided presacral lesion abutting the S1 nerve root (Figure 7A,B,C,D). The patient underwent en bloc resection via a midline anterior approach. The patient had an uneventful post-operative course and remains neurologically nonfocal without evidence of recurrence at 12-month follow-up (Figure 7E,F,G).
Description of techniques
Robot-assisted anterior endoscopic approach (case 1)
The patient is placed supine in the lithotomy position and a Veress needle is used to insufflate the peritoneal cavity. A 12 mm trocar is placed through the umbilicus to visualize the intraabdominal contents and aid placement of three 8 mm trocars. Trendelenburg positioning is used to retract the abdominal viscera and the surgical robot is docked to the table. The peritoneum is incised over the mass and resected medially and laterally, exposing the ureter and iliac vessels, which are identified and protected. The rectum is reflected laterally using the medial peritoneal flap and in females, the uterus and adnexa are reflected laterally, creating a corridor to access the tumor. The mass is stimulated to rule out involvement of somatic motor nerves, and then a plane is developed around the tumor capsule, taking care to dissect away any viable nerve roots. The tumor is then reflected off the ventral sacrum and mobilized. It is placed into an endocatch bag and morcellated for delivery through the 12 cm umbilical trocar. The presacral fascia is then closed and a drain is placed through the lateral 8 mm trocar port; port sites are closed with subcuticular 4-0 Biosyn suture.
Posterior open approach with instrumentation (case 5)
The patient is placed prone and draped and a posterior midline incision is formed from the distal sacrum to the top vertebra to be instrumented. Conventional approach through the avascular plane is made with subperiosteal dissection extending laterally to the facet joints of the lumbar spine and medial aspects of the iliac wings, allowing complete visualization of the tumor. Multilevel sacral laminectomy is performed, as well as lumbar laminectomy at all levels with an intracanalicular component to expose and free the thecal sac. Pedicle screw and pelvic instrumentation is then placed (when indicated). The tumor is then debulked until gross total resection has been achieved. If significant nerve root involvement is noted, subtotal resection may be chosen (i.e., lumbar or S1 root involvement) or the involved nerve may be sacrificed. Plastic surgery may assist with closure by performing paraspinous muscle flaps with musculofascial advancement of the gluteus maximus.
Anterior open approach (case 7)
The patient is placed supine and midline laparotomy is performed with the assistance of an access surgeon. The sigmoid colon is mobilized from the presacral space to expose the tumor mass. The ureters are identified bilaterally and protected. A plane is then established between the tumor mass and the presacral alar tissue. Next, small feeding vessels are individually coagulated and transected to further mobilize the tumor. In circumferential fashion the tumor is then completely mobilized from the sigmoid colon, the left iliac vein and other surrounding anatomy. Finally, the remaining attachment of the tumor tissue to the presacral nerve root(s) is identified and carefully dissected free with bipolar cautery. The tumor is then completely mobilized and delivered en bloc from the surgical field. The wound is then closed in standard fashion.
Conclusions
Presacral schwannomas are indolent masses that may be confused with other pelvic and presacral tumors, including malignant vertebral column tumors (e.g., chordoma), congenital tumors (teratoma), and soft tissue sarcomas (12). Current standard of care for these lesions is surgical resection, with the goal of relieving both pain secondary to nerve root compression and bulk symptoms (e.g., constipation) resulting from compression of the surrounding viscera. Because of the intimate relation of the tumor to the sacral nerve roots, surgery may be complicated by sacral nerve root damage and subsequent bowel, bladder, or sexual dysfunction, and occasionally motor weakness. This sequela is most likely with damage to the S1 or S2 roots, with the former increasing the risk of post-operative weakness (13).
The gold standard for identification is a pelvic MRI, which demonstrates a T2 hyperintense, T1-hypointense mass with smooth, well-defined margins and enhancement on post-contrast fat suppressed T1-weighted sequences. Histological confirmation is made by documenting eosinophilic, spindle-shaped cells divided into cellular Antoni-A and hypocellular Antoni-B regions (14). Well-developed or “ancient” schwannomas often demonstrate cystic changes secondary to stromal and vasculature degeneration, demonstrable as non-enhancing T2-hyperintense fluid pockets (11,14).
To date, more than 130 articles describing over 220 presacral schwannomas have been reported in the literature, including the seven cases presented here (author literature search—18th October 2018). Roughly 40% of reported lesions have been treated via open anterior approaches and 35% via posterior approaches, with the remaining 25% split evenly between staged anterior-posterior and endoscopic anterior approaches. The approach selected is dictated by surgeon experience as well as the morphology of the lesion.
Two systems have been previously published to describe the morphology of these lesions—those of Sridhar and Klimo (14,15). The earlier Sridhar system was developed to describe all spinal schwannomas with intracanalicular components: type I lesions were purely intraspinal and less than 2 vertebral segments in length, type II were purely intraspinal but greater than 2 segments in length, type III were intraspinal with foraminal involvement, type IV were conventional dumbbell tumors without vertebral body erosion, and type V were the most invasive dumbbell tumors, possessing vertebral body erosion (15). Klimo and colleagues then consolidated this system into three groups: purely sacral/intracanalicular (type I), purely presacral (type III), or mixed intracanalicular/presacral (type II) (14). For all of these lesions, Klimo and others suggest gross total resection, with several suggesting that recurrence risk is decreased with en bloc resection. However, a R0 en bloc resection may have unacceptable morbidity depending upon tumor morphology, surgeon skill, and patient anatomy.
Conventionally, Klimo type I lesions are treated with posterior approaches, as this allows for direct visualization of the cauda equina and exiting sacral roots while resecting the tumor. Klimo type III lesions by contrast have traditionally been approached via a transabdominal approach, as this reduces the risk to the sacral roots and facilitates en bloc delivery of the lesion, as described in case 7 of this series. Extremely large, type III lesions may not be amenable to en bloc resection due to anatomic constraints imposed by the pubic symphysis and bladder, which can obfuscate the inferior pole of the lesion, as in case 6. In such cases, safe dissection requires internal debulking (e.g., with an ultrasonic aspirator), followed by capsular dissection. Only circumstantial evidence exists comparing relative rates of recurrence between gross total resection achieved via piecemeal and en bloc approaches (14). Given the benign nature of these lesions, it is advisable to employ debulking followed by capsule delivery unless all margins of the lesion can be visualized (compare cases 6 and 7). Finally, Klimo type II lesions often require staged anterior-posterior approaches. In our series, we elected to employ posterior-only approaches. In cases 2, 3, 5 and 6 we achieved gross-total resection and saw no evidence of local disease recurrence, suggesting that a posterior-only approach is a valid option for Klimo type II lesions. In case 4, we were only able to achieve subtotal resection via the posterior approach owing to the substantial tumor size and involvement of numerous sacral roots. Because the S1 roots were involved, a complimentary anterior approach was not offered as gross total resection was felt to be impossible and a second approach would needlessly increase operative morbidity without altering recurrence risk.
Recently, additional innovations have been implemented for the surgical treatment of presacral schwannomas. Notably, Yin et al. published a series of 5 patients treated with robot-assisted endoscopic anterior approaches (16). In all cases en bloc resection was achieved, as in case 1 of the present series. Blood loss in this series was substantially lower than that reported in other series of presacral schwannoma as well as compared to the posterior-only approaches reported here. Given that the surgeon has sufficient experience with the robotic device and the patient possesses body habitus conducive to the anterior approach, robot-assisted endoscopic resection may be an option for Klimo type III lesions, especially in patients with extensive medical comorbidities that render them suboptimal candidates for open approaches.
In summary, sacral and presacral schwannomas are rare clinical entities that can be safely approached with anterior or posterior approaches. The relative merits of the two options are largely dictated by the extent of bony and sacral nerve root involvement. Higher-quality evidence is needed to compare outcomes of anterior and posterior approaches for treatment of these lesions.
Acknowledgments
None.
Footnote
Conflicts of Interest: M Goodwin is a Consultant for ROM3, Augmedics; DM Sciubba is a Consultant for Medtronic, Depuy-Synthes, Globus, K2M, Stryker, Baxter, Nuvasive. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Celli P, Trillò G, Ferrante L. Spinal extradural schwannoma. J Neurosurg Spine 2005;2:447-56. [Crossref] [PubMed]
- Jeon JH, Hwang HS, Jeong JH, et al. Spinal schwannoma; analysis of 40 cases. J Korean Neurosurg Soc 2008;43:135-8. [Crossref] [PubMed]
- Seppälä MT, Haltia MJ, Sankila RJ, et al. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg 1995;83:621-6. [Crossref] [PubMed]
- Safaee MM, Lyon R, Barbaro NM, et al. Neurological outcomes and surgical complications in 221 spinal nerve sheath tumors. J Neurosurg Spine 2017;26:103-11. [Crossref] [PubMed]
- Lenzi J, Anichini G, Landi A, et al. Spinal Nerves Schwannomas: Experience on 367 Cases-Historic Overview on How Clinical, Radiological, and Surgical Practices Have Changed over a Course of 60 Years. Neurol Res Int 2017;2017:3568359. [Crossref] [PubMed]
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol 2012;123:295-319. [Crossref] [PubMed]
- Theodosopoulos T, Stafyla VK, Tsiantoula P, et al. Special problems encountering surgical management of large retroperitoneal schwannomas. World J Surg Oncol 2008;6:107. [Crossref] [PubMed]
- Gupta S, Sikora SS, Gupta R, et al. Presacral neurilemoma (schwannoma)--report of a rare case. Jpn J Surg 1989;19:229-31. [Crossref] [PubMed]
- Andonian S, Karakiewicz PI, Herr HW. Presacral cystic schwannoma in a man. Urology 2003;62:551. [Crossref] [PubMed]
- Lee BH, Hyun SJ, Park JH, et al. Single Stage Posterior Approach for Total Resection of Presacral Giant Schwannoma: A Technical Case Report. Korean J Spine 2017;14:89-92. [Crossref] [PubMed]
- Ozturk C, Mirzanli C, Karatoprak O, et al. Giant sacral schwannoma: a case report and review of the literature. Acta Orthop Belg 2009;75:705-10. [PubMed]
- Makni A, Fetirich F, Mbarek M, et al. Presacral schwannoma. J Visc Surg 2012;149:426-7. [Crossref] [PubMed]
- Schwab JH, Healey JH, Rose P, et al. The surgical management of sacral chordomas. Spine (Phila Pa 1976) 2009;34:2700-4. [Crossref] [PubMed]
- Klimo P Jr, Rao G, Schmidt RH, et al. Nerve sheath tumors involving the sacrum. Case report and classification scheme. Neurosurg Focus 2003;15:E12. [Crossref] [PubMed]
- Sridhar K, Ramamurthi R, Vasudevan MC, et al. Giant invasive spinal schwannomas: definition and surgical management. J Neurosurg 2001;94:210-5. [PubMed]
- Yin J, Wu H, Tu J, et al. Robot-assisted sacral tumor resection: a preliminary study. BMC Musculoskelet Disord 2018;19:186. [Crossref] [PubMed]


