
Clinicopathological characteristics and treatment strategies for young lung cancer patients
Introduction
Lung cancer is one of the leading causes of cancer-related deaths worldwide regardless of sex (1). In the US, it is a particularly prominent disease in the elderly population; people <45 years of age account for <2% of all cases of lung cancer (2). According to the Japanese Ministry of Health, Labor, and Welfare, an estimated 146,000 people suffered from respiratory cancer and 73,396 deaths were attributed to respiratory cancer in 2014. According to a Japanese Lung Cancer Registry Study in 2004, patients younger than 50 years (data available since 1994) (3) accounted for between 5.0% and 8.2% of all lung cancer patients who underwent surgical resection. Several studies have shown that post-therapeutic survival rates in younger patients with lung cancer are higher than those in their older counterparts (4-6); however, other studies have shown comparable survival outcomes among patients in different age groups (7,8). Some investigators have suggested that the course of lung cancer in young patients is more aggressive than that in older patients (9). Owing to the small number of young patients with lung cancer, their clinicopathological characteristics are not well characterized; moreover, there is no consensus on the most effective management strategies for these patients. Therefore, this study aimed to elucidate the clinicopathological characteristics and the best therapeutic strategies for lung cancer in young patients and compared them with elderly patients.
Methods
Patient selection
We retrospectively reviewed 4,733 consecutive patients with pathologically confirmed lung cancer at the Tokyo Medical University Hospital from January 2000 to December 2014. Of these, 36 patients who did not undergo therapeutic management were excluded. The remaining 4,697 patients were divided into the following three groups by age: <50 years (n=266), between 50 to 69 years (n=2,360), and ≥70 years (n=2,071). The diagnosis and treatment of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) were performed in accordance with the then guidelines of the Japan Lung Cancer Society. The protocols for data collection and analyzes were approved by the institutional review board of the Tokyo Medical University. The requirement for informed consent from the patients was waived off due to the retrospective nature of the study. Confidentiality of patient data was maintained throughout the study.
Data collection
Pretreatment evaluation and treatment management included physical examination, blood examination, chest radiography, and chest and abdomen computed tomography (CT). Brain CT or magnetic resonance imaging and positron emission tomography-CT were performed if clinically indicated. Staging and pathological findings for lung cancer were determined according to the seventh TNM Classification for Lung and Pleural Tumors (10) and the World Health Organization classification (11). The hospital charts of all patients were reviewed to collect clinicopathological data, including age; sex; smoking history; clinical T, N, and M factors; histological type; surgical procedures; epidermal growth factor receptor (EGFR) mutation status; and overall survival (OS). OS was determined as the duration from the day of initial diagnosis until the day of death from all causes. Data pertaining to patients who were alive and showed no evidence of recurrence at the end of the follow-up period were censored from the analysis.
Statistical analysis
Categorical variables are presented as frequencies and proportions, whereas continuous variables are presented as mean ± standard deviation. Patient characteristics were compared using the chi-squared test or Fisher exact test for categorical outcomes of continuous variables, as appropriate. OS was estimated using the Kaplan-Meier method, and between-group differences were assessed with log-rank test. P values and hazard ratios in the multivariate analyses were calculated using the Cox regression model. Between-group differences associated with P values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS statistical software (version 21.0; SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
Clinicopathological characteristics of patients with lung cancer from 2000 to 2014, disaggregated by age groups (≤49, 50–69, and ≥70 years) are shown in Table 1. In the ≤49 years age group, there were 169 (63.5%) men and 97 (36.5%) women; no significant between-group difference was observed with respect to sex distribution (P=0.809). Approximately 80% of patients with therapeutic lung cancer in the ≤49 and 50–69 years age groups had performance status 0–1, which was better than that of patients in the ≥70 years age group. A majority of the patients in all three groups were consulted us because of an abnormal chest shadow detected on routine health checkup. The proportion of “never smokers” in the ≤49 years age group (36.5%) was significantly higher than that in the other age groups. The proportion of patients with stable disease in the ≤49 years age group ranged from 5.4% to 5.9%. Regarding clinical stage, 125 patients in the ≤49 years age group had advanced stage tumors at presentation, which exhibited a trend of being higher than that in the other groups (P=0.053). The percentage of adenocarcinoma and carcinoid tumors was significantly higher in the group aged ≤49 years whereas that of squamous cell carcinoma was significantly lower compared to the other groups. Squamous cell carcinoma was higher in the group aged ≥70 years than in the younger groups. Furthermore, we analyzed EGFR mutation status in a limited number of patients; the rate of patients with an EGFR mutation (particularly exon 19 deletion) in the ≤49 years age group was significantly higher than that in the other groups (12). Regarding initial therapeutic strategies, patients in the ≥70 years age group underwent more nonsurgical treatments as compared with those in the younger age groups (P=0.0001). Subsequently, sublobar resection and other less invasive surgical procedures were more frequently selected in these patients (P=0.0001).
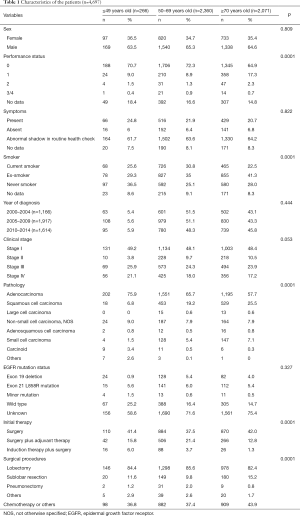
Full table
Survival
The median follow-up time of all patients was 43.1 months (range, 0.3–189.0 months). The median follow-up time in the ≤49, 50–69, and ≥70 years age groups was 46.3, 46.6, and 38.7 months, respectively. The median follow-up time in the ≥70 years age group was significantly lower than that in the ≤49 and 50–69 years age groups (P=0.001 and P=0.045). The 1-, 3-, and 5-year survival rates in the ≤49 years age group were 82.9%, 64.6%, and 57.0% (Figure 1A). The 1-, 3-, and 5-year survival rates of patients in the ≤49 years age group who underwent surgery were 97.6%, 88.2%, and 81.5%, respectively (Figure 1B). The 1-, 3-, and 5-year survival rates of patients in the ≤49 years age group who did not undergo surgery were 55.9%, 21.1%, and 12.8%, respectively (Figure 1C).
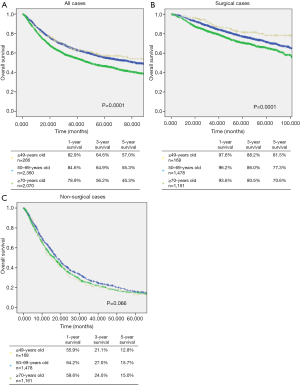
The postoperative survival rate in the ≤49 age group was significantly higher than the other 50–69 and ≥70 years age groups (P=0.29 and P<0.0001, respectively) (Figure 1B). In the entire study population, although the survival rate in the ≤49 years age group was significantly higher than that in the ≥70 years age group (P=0.0001), it was not significantly different from that in the 50–69 years age group (P=0.439) (Figure 1A). Moreover, among patients who did not undergo surgery, the survival rate of those in the ≤49 years age group was not significantly different from the survival rates of those in the other age groups (P=0.066) (Figure 1C).
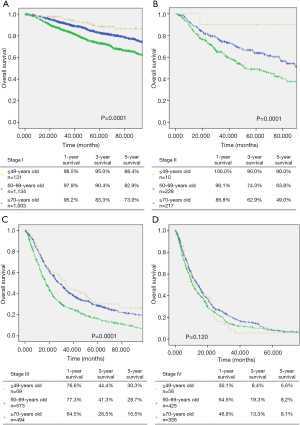
The detailed survival data of patients with NSCLC of different TNM stages are summarized in Figure 2. From the point of clinical staging, 1-, 3-, and 5-year survival rates of patients with stage I lung cancer in the ≤49 years age group were 98.5%, 95.0%, and 88.4%, respectively (Figure 2A). The 1-, 3-, and 5-year survival rates of patients with stage II lung cancer in the ≤49 years age group were 100.0%, 90.0%, and 90.0%, respectively (Figure 2B). The 1-, 3-, and 5-year survival rates of patients with stage II lung cancer in the ≤49 years age group were 76.6%, 44.4%, and 30.3%, respectively (Figure 2C). The 1-, 3-, and 5-year survival rates of patients with stage IV lung cancer in the ≤49 years age group were 50.1%, 8.4%, and 5.6%, respectively (Figure 2D). The OS rates of lung cancer patients with clinical stages I, II, and III in the ≤49 years age group were better than those of their counterparts older than 50 years.
Regarding sex differences, the 1-, 3-, and 5-year OS rates in females were higher than that in males (Figure 3A).
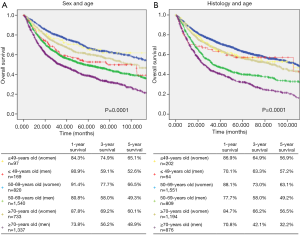
The 1-, 3-, and 5-year OS rates of patients with adenocarcinoma were better than that of patients with non-adenocarcinoma. In the non-adenocarcinoma group, the 3- and 5-year OS rates of younger patients were higher than those of older patients (Figure 3B).
Discussion
Lung cancer mostly occurs in patients of older age (13). Therefore, lung cancer in young patients is recognized to be relatively rare and to comprise a distinct oncological entity.
Owing to the reported increase in the incidence of lung cancer among young patients in Europe and Japan (14,15), there is an urgent need to characterize young lung cancer patients. Several studies have compared the clinicopathological features and prognoses of young NSCLC patients of different ages (9,16-19). In general, epidemiological observations have been explained by the time period required for the environmental toxins to induce genetic damage that ultimately leads to cancer. Therefore, many studies have suggested that lung cancer in the young may constitute a distinct clinicopathological entity with a different sex distribution, stage at diagnosis, pathological features, and prognosis, although the reported data are frequently discordant (3,8,9,16-20).
At our institution, a total of 266 patients in the ≤49 years age group were registered between 2000 and 2014, which accounts for 5.7% of all cases (Table 1). This is consistent with a previous nationwide Japanese cohort (6) that was stable over the last 15 years. The young patients included 169 men and 97 women; the male:female ratio of 1.74:1 (Table 1) is similar to that in a Japanese nationwide cohort reported in 2004 (3), and comparable with that in the older age groups in the present study. We also found that the histological type in almost 80% of the younger patients was adenocarcinoma (Table 1). It is possible that there are less cases of smoking-related squamous carcinoma or small cell carcinoma in young patients.
The therapeutic strategy is sometimes constrained in older patients because of existing comorbidities, which may not be the case with younger patients.
Regarding the clinical TNM staging of young lung cancer patients in this series, there were 131 stage I, 10 stage II, 69 stage III, and 56 stage IV patients; the proportion of patients with stages III–IV advanced lung cancer was higher than that in the older age groups (P=0.053) (Table 1). In comparison with elderly patients, it has suggested that lung cancer in young patients tends to be more aggressive in nature; however, the performance status of these young patients tends to be better (6,19,21,22).
The present study suggests that surgical resection was more frequently selected as the first-line treatment for younger patients as compared with that in the other age groups. The higher rates of neoadjuvant chemotherapy in the younger group may reflect the higher proportion of patients with operable stage III advanced disease, which is a prime indication for induction therapy (6); moreover, young patients tend to show better tolerance for such treatment owing to better functional status of organs. The rate of neoadjuvant chemotherapy in the younger group was more than two-fold higher than that in the older group. Radzikowska et al. also reported similar results with more aggressive treatment in younger patients (4). These results indicate that planned active multimodal strategies in younger patients result in good performance status. In addition, among patients who underwent surgical resection, the 5year survival rates in the ≤49 years age patients group was the highest at 81.5%; the corresponding rates in the 50–69 and ≥70 years age groups were significantly lower at 77.3% and 70.6%, respectively (P=0.0001) (Figure 1B). Therefore, more aggressive multimodality treatments, including surgery, are feasible for young patients with lung cancer and are highly recommended in our daily practice.
The tendency for a higher survival rate in female patients and in patients with adenocarcinoma was similar to that seen with the analyses of all ages (6) (Figure 3). Adenocarcinoma in situ showing ground-glass opacity on CT, which is currently classified as adenocarcinoma in situ or minimally invasive adenocarcinoma (23), is generally considered as a slow-growing, low-grade adenocarcinoma and a unique subtype associated with a never-smoking history and female sex (24). Although CT data were not analyzed in the present study cohort, higher proportions of adenocarcinoma and females in the young patient group might affect the patients' characteristics, with a higher rate of adenocarcinoma in situ with lower malignant behavior.
Limitations
This single institutional study of a large number of surgically and non-surgically treated lung cancer patients has several limitations. As it is a retrospective study, we cannot clarify the prognostic effects of multimodal therapy in young patients owing to limited comprehensive data, including data pertaining to chemotherapy regimens or molecular targeted therapy during their entire treatment course. Other limitations are the persistence of patient-selected treatment bias and time-to-detection bias that may have worsened prognostic outcomes in elderly patients. Moreover, we could not analyze the treatment outcomes after recurrence in the present study; young patients with good performance status might have more chance to receive secondary or more chemotherapy in combination with radiation or immunotherapy. Further prospective, multi-institutional investigations and substantial clinical analyzes are required to clarify the cause underlying the better prognosis of younger patients who receive treatment as compared with patients in the other age groups (25).
Conclusions
We retrospectively reviewed 4,697 consecutive patients with pathologically confirmed lung cancer who received surgical and nonsurgical treatment at the Tokyo Medical University Hospital between January 2000 and December 2014. We compared the clinicopathological characteristics and survival outcomes of younger patients and older patients.
We interestingly found better postoperative survival of these young lung cancer patients, although the proportion of patients with advanced disease was higher compared with that in the older group.
Clinical practice points
- Especially to postoperative patients, the survival rate is better than older group.
- Longer-term follow-up is essential for primary cancer and associating health care problems (26-28).
- Data storage system including specimen sample is needed expecting to treatment options in future.
Acknowledgements
Medical English writing assistance was provided by Crimson Interactive Pvt. Ltd. The authors are fully responsible for the content and editorial decisions for this manuscript. This study was supported by a Grant-in-Aid for Scientific Research, from the Japan Society for the Promotion of Science, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocols for data collection and analyzes were approved by the institutional review board of the Tokyo Medical University. The requirement for informed consent from the patients was waived off due to the retrospective nature of the study.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Key Statistics for Lung Cancer. Available online: https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html, Accessed 05.29, 2017.
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Radzikowska E, Roszkowski K, Glaz P. Lung cancer in patients under 50 years old. Lung Cancer 2001;33:203-11. [Crossref] [PubMed]
- Tian DL, Liu HX, Zhang L, et al. Surgery for young patients with lung cancer. Lung Cancer 2003;42:215-20. [Crossref] [PubMed]
- Inoue M, Okumura M, Sawabata N, et al. Clinicopathological characteristics and surgical results of lung cancer patients aged up to 50 years: the Japanese Lung Cancer Registry Study 2004. Lung Cancer 2014;83:246-51. [Crossref] [PubMed]
- Hanagiri T, Sugio K, Uramoto H, et al. Results of surgical treatment for lung cancer in young adults. Int Surg 2008;93:50-4. [PubMed]
- Maruyama R, Yoshino I, Yohena T, et al. Lung cancer in patients younger than 40 years of age. J Surg Oncol 2001;77:208-12. [Crossref] [PubMed]
- Minami H, Yoshimura M, Matsuoka H, et al. Lung cancer treated surgically in patients <50 years of age. Chest 2001;120:32-6. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C, et al. editors. TNM classification of malignant tumours. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell, 2010:310.
- Travis W, Brambilla E, Müller-, and Müller-Hermelink H. World Health Organization Classi-fication of Tumours: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004.
- Wang Y, Chen J, Ding W, et al. Clinical Features and Gene Mutations of Lung Cancer Patients 30 Years of Age or Younger. PLoS One 2015;10:e0136659. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
- Strand TE, Malayeri C, Eskonsipo PK, et al. Adolescent smoking and trends in lung cancer incidence among young adults in Norway 1954-1998. Cancer Causes Control 2004;15:27-33. [Crossref] [PubMed]
- Marugame T, Yoshimi I, Kamo K, et al. Trends in lung cancer mortality among young adults in Japan. Jpn J Clin Oncol 2005;35:177-80. [Crossref] [PubMed]
- Sugio K, Ishida T, Kaneko S, et al. Surgically resected lung cancer in young adults. Ann Thorac Surg 1992;53:127-31. [Crossref] [PubMed]
- Bourke W, Milstein D, Giura R, et al. Lung cancer in young adults. Chest 1992;102:1723-9. [Crossref] [PubMed]
- Capewell S, Wathen CG, Sankaran R, et al. Lung cancer in young patients. Respir Med. 1992;86:499-502. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Thomas A, Chen Y, Yu T, et al. Trends and Characteristics of Young Non-Small Cell Lung Cancer Patients in the United States. Front Oncol 2015;5:113. [Crossref] [PubMed]
- Zhang J, Chen SF, Zhen Y, et al. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer 2010;116:3656-62. [Crossref] [PubMed]
- Liu M, Cai X, Yu W, et al. Clinical significance of age at diagnosis among young non-small cell lung cancer patients under 40 years old: a population-based study. Oncotarget 2015;6:44963-70. [Crossref] [PubMed]
- Saji H, Matsubayashi J, Akata S, et al. Correlation between whole tumor size and solid component size on high-resolution computed tomography in the prediction of the degree of pathologic malignancy and the prognostic outcome in primary lung adenocarcinoma. Acta Radiol 2015;56:1187-95. [Crossref] [PubMed]
- Kudo Y, Matsubayashi J, Saji H, et al. Association between high-resolution computed to-mography findings and the IASLC/ATS/ERS classification of small lung adenocarcinomas in Japanese patients. Lung Cancer 2015;90:47-54. [Crossref] [PubMed]
- Coccia PF, Pappo AS, Beaupin L, et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:66-97. [Crossref] [PubMed]
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500-10. [Crossref] [PubMed]
- Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991-4008. [Crossref] [PubMed]
- Johannsdottir IM, Loge JH, Kiserud CE, et al. Increased prescription rates of anxiolytics and hypnotics to survivors of cancer in childhood, adolescence, and young adulthood-A population-based study. Pediatr Blood Cancer 2018;65:e26860. [Crossref] [PubMed]


