
Mind the gap: renal tubule responses to injury and the role of Cxcl12 and Myc
Acute kidney injury (AKI) is a common disorder resulting from multi-factorial ischemic, toxic and septic insults to the kidney (1,2). A common feature of all of these injuries is that there is often a profound reduction in renal blood flow resulting in tissue hypoxia. Therapeutic options are limited and there is considerable interest in understanding the response of the kidney to injury so that better interventions can be developed. In a study published in Nature Communications, Yakulov et al. utilize zebrafish and mouse models of AKI to show that the renal tubules undergo a metabolic switch to glycolysis as an adaptive and protective response to injury. By studying genetic knockouts and transgenic animals, combined with RNA-Seq, they implicate the chemokine ligand Cxcl12 (aka Sdf1) and its G protein-coupled receptor Cxcr4, as well as the proto-oncogene Myc, as key responders to injury and potential drivers of metabolic reprogramming (3).
Yakulov et al. begin by ablating short stretches of the zebrafish pronephric (embryonic kidney) tubules using a 2-photon laser. The zebrafish is ideally suited for these types of experiments as the pronephros can be readily visualized in vivo (4,5). Using time-lapse imaging, they confirm the previous findings of Palmyre et al. that showed that collective cell migration drives restoration of tubule integrity (6). Specifically, cells bordering the ablated region extend protrusions and actively migrate towards each other to repair the lesion. Such migratory processes are not just reserved for injury responses but occur in two waves normally during the development of the zebrafish pronephros. The first wave occurs soon after the tubule has epithelialized (14–20 hours post-fertilization; hpf), resulting in a posterior-directed migration that most likely drives the fusion of the tubules to the cloaca (5). From 28 hpf onwards, tubular cell movement reverses direction and the cells move anteriorly in response to fluid flow down the tubule (7,8). Intriguingly, Yakulov et al. found a temporal switch in the type of tubule repair response. While complete tubule repair is achieved when ablation occurs after 36 hpf, ablations induced before 30 hpf fail to repair. Instead, actomyosin bundles accumulate adjacent to the site of injury, consistent with the ends of the tubule being sealed via ‘purse string’ occlusions like those seen during embryonic wound healing (9). The cause for this developmental switch in repair mechanisms is not known. However, during the fusion of the tubule to the cloaca in the first migratory wave, the distal-most tubule cells exhibit an active migratory behavior that is similar to that seen during the repair at later stages (10). This raises the possibility that prior to 30 hpf there are suppressive mechanisms that restrict migratory behavior along the tubule except for the distal-most cells involved in cloacal fusion.
In the next step, Yakulov and colleagues ruled out a role for fluid flow, Wnt signaling (canonical and non-canonical), par6b (a component of the apico-basal polarity complex) and dedifferentiation as drivers of the migratory repair process. Turning to the unbiased approach of RNA-Seq, the authors compared micro-dissected tubules from non-regenerating (24 hpf) and regenerating (48 hpf) stages and together with insights from the ZFIN expression database, they selected cxcl12a and myca as candidate regulators. Cxcl12 is best known for its chemotactic effects on a range of cells including blood, vascular and the posterior lateral line cells in zebrafish (11), while Myc is a transcription factor that regulates a host of genes involved in growth and metabolism (12). Cells proximal to the site of injury were found to upregulate expression of myca and cxcr4b, whereas cxcl12a appears to be constitutively expressed in the tubules.
Using knockdown animals, Yakulov et al. demonstrate that tubule repair is perturbed in ~60% of cxcr4b−/−, ~40% of cxcl12a−/− and 25% of myca−/− embryos. Some of the cxcl12a−/−embryos showed aberrant repair that manifested as a dorsal out-pouching of the epithelium, suggestive of wayward migration. In addition, ectopic expression of cxcl12a under the control of a heat-shock inducible promoter also perturbed repair. Together, these results support a role for the Cxcl12a-Cxcr4b axis in coordinating the migratory repair response, presumably involving a chemotactic gradient of Cxcl12a, although further clarification is needed. An interesting observation of the migratory repair response is that tubule cells at the anterior end of the lesion must migrate posteriorly, thus reversing their normal posterior-to-anterior migration that is associated with the second migratory wave. Yakulov and colleagues found that in myca-deficient animals this reversal failed to happen, leading to the suggestion that myca may act to transiently override the directionality of the second migratory wave.
To extend the findings from zebrafish into mammals, the authors next investigated if tubule-specific knockout of Cxcl12 and Myc in mice influenced the severity of ischemia/reperfusion (IR)-induced AKI. Unlike the more simple ablation experiments in zebrafish, IR-AKI in mammals is a much more complicated model with several temporal phases that involve structural and functional changes to multiple cell types in the kidney and a significant involvement of the immune system (13). Yakulov et al. focus on the very early phase of injury (12 hours post-IR) when kidney cells are dealing with the pathological follow-on effects of the ATP depletion induced by transient hypoxia. In particular, reduced functioning of the ATP-dependent Na+/K+ ATPase results in intracellular Na+ retention, a corresponding influx of water and cell swelling. Other early post-IR changes include local interstitial edema, increases in blood viscosity and coagulation, and vascular alterations such as vasoconstriction and immune cell recruitment. Together, these pathological changes result in a prolonged impairment in renal blood flow with a corresponding decrease in kidney filtration function and continued tissue damage (13).
Yakulov et al. found that mice with a tissue-specific knockout of Cxcl12 or Myc in the renal tubules exhibit higher serum urea levels and higher urinary excretion of the injury marker Ngal following IR-AKI, indicating that these genes help preserve kidney function during the early phases of injury. By performing RNA-Seq on the kidneys, they discovered that the knockout mice display reduced expression of metabolic and mitochondria-associated genes. In addition, by performing a metabolomic analysis of urine samples they found decreased glucose and lactate in the urine from the knockout animals, leading the authors to hypothesize that gluconeogenesis and glycolysis is compromised in Cxcl12- and Myc-deficient animals upon IR-AKI. The authors directly tested this in the case of Myc-deficient mice by measuring the extracellular acidification rate in primary tubular epithelial cells and found reduced glycolytic flux in the knockout cells. This result is in keeping with the long established role of Myc as a stimulator of glycolysis (14). The involvement of Cxcl12-Cxcr4 signaling in glycolysis is less well understood but a prior report has shown that interfering with this axis in cultures of leukemic cells reduces glucose uptake and glycolysis (15). Together, these observations suggest that Myc and Cxcl12 act to stimulate glycolysis and upregulate the expression of mitochondrial genes in renal tubule cells during IR-AKI. These metabolic adaptations might serve to offset the ATP depletion induced by the ischemia and help reduce tubular cell injury. Indeed, earlier work has shown that there is a linear correlation of the 2-hour total kidney ATP level and the degree of renal function at 24 hours post-IR (16).
Presumably in injured Cxcl12- and Myc-deficient animals the greater tubule dysfunction translates into lowered kidney filtration function via more severe hemodynamic changes but this was not directly assessed. Instead, Yakulov and colleagues identify from the RNA-Seq data that Cxcl12- and Myc-deficient kidneys show differential expression of genes involved in the retinoic acid (RA) pathway. A recent study has linked RA to IR-AKI, with RA signaling increasing within hours of IR and acting to suppress pro-inflammatory (M1 spectrum) macrophages (17). Macrophages are recruited into the injured kidney as early as 1-hour post-IR and pro-inflammatory macrophages make a major contribution to the propagation of tissue injury in IR-AKI (18). RA operates in multiple cells in the injured kidney but relevant to the Yakulov study is that RA signaling in proximal tubule cells is involved in promoting anti-inflammatory (M2 spectrum) macrophages in mouse kidneys after IR-AKI (17). This effect is likely mediated via the RA-induced secretion of as yet unidentified factors that promote the polarization of macrophages to an M2 spectrum phenotype. Although Yakulov et al. did not examine this pathway or the involvement of macrophages, their findings suggest that Cxcl12 and Myc may act as upstream regulators of RA signaling in injured tubule cells during IR. The importance of RA to the injury and loss of kidney function observed in the early stages of IR-AKI is not clear as Chiba et al. (17) found that inhibition of RA signaling has only limited effects on tubular injury and no effect on renal function in IR-AKI (despite exacerbating post-injury fibrosis at later stages). This contrasts with exogenous administration of RA, which both Yakulov et al. (3) and Chiba et al. (17) found improves renal function during IR-AKI, although non-physiological doses were used making it difficult to interpret these effects. Putting all the data together, a model can be constructed whereby during the early stages of IR-AKI, Cxcl12 and Myc act to promote glycolysis and RA signaling in tubule cells and in doing so, offset the alterations in cell energetics caused by the hypoxia and promote the polarization of macrophages to an anti-inflammatory phenotype, respectively (Figure 1). These adaptations are expected to help preserve kidney function during IR-AKI by reducing damage/swelling to the tubules and restricting inflammatory processes that lead to a reduction in renal blood flow.
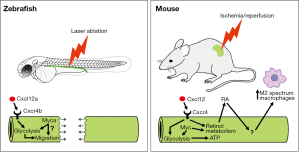
At first glance the zebrafish and mouse data appear quite distinct, and indeed the types of injury and cellular responses are very much model specific. Despite this, pathways such as RA that were originally discovered in the zebrafish model to promote recovery from AKI are also used in the mouse, albeit operating via different mechanisms (17). In the zebrafish, tubule repair involves a non-proliferative, migratory mechanism that restores tubule integrity (6). In the mouse, tubule repair is not actually examined by Yakulov and colleagues, as this initiates 2−3 days post-IR and involves proliferation of surviving tubule cells (19). Instead, the mouse experiments are focused on early stages of injury where cellular and hemodynamic dysfunction causes a loss of kidney function. However, Yakulov et al. link the two discrete processes together by showing that a glycolytic switch in metabolism is important in both contexts. The authors do this by going back to the zebrafish and demonstrating that chemical inhibition of glycolysis delays the migratory repair process, although it does not prevent it completely. They conclude that anaerobic energy production stimulated by the Cxcl12 and Myc pathways may be important for enabling the fast cell movements that occur during tubule repair. This notion fits with a recent report showing that glycolysis is critical for the cell motility needed to extend the axis of mouse and chick embryos (20). An attractive hypothesis is that two processes are simultaneously activated when the zebrafish tubule is damaged: the first being a switch to glycolysis to generate a fast supply of ATP and to redirect glycolytic intermediates towards the increased anabolic reactions needed to drive fast cell migration, and the second being the stimulation of collective cell migration (Figure 1).
The authors’ work highlights important questions for further research. Are Cxcl12 and Myc operating in common or parallel pathways? Myc has been shown to upregulate the Cxcr4 promoter in vitro (21) and Yakulov et al. mention that overexpression of cxcr4b can partially rescue the myca-deficient repair defect in zebrafish. However, cxcr4b expression is unaffected in zebrafish myca mutants. Thus, the epistatic relationship between the Cxcl12-Cxcr4 axis and Myc is unclear. What is the connection of Cxcl12 and Myc to the immune response following IR injury in the mouse? Yakulov and colleagues focused on the 12 hours post-IR stage when macrophages and other immune cells are being recruited into the injured kidney but tubular repair has not initiated. Given the established role of Cxcl12 on immune cell chemotaxis and the prior work of RA on modulating macrophage activation it will be essential to follow-up this study with a focus on how Cxcl12 and Myc deficiency affects the immune/inflammatory response following kidney injury. What is the long-term effect of Cxcl12 and Myc deficiency on tubule repair in mammals? By extending out the analysis of IR-AKI in Cxcl12 and Myc deficient mice by 3-4 weeks, the role of these genes during tubule repair and post-injury fibrosis could be assessed. These studies should address whether tubular cell proliferation and apoptosis are affected, given that both the Cxcl12-Cxcr4 axis and Myc have been implicated in these processes. How does the notion that glycolysis plays a protective role during IR-AKI fit with the recent report by Zhou et al. that suggests the opposite is true (22)? Zhou et al. show that nitric oxide inhibits glycolysis causing excess glucose to be shunted into the pentose phosphate pathway, thereby generating high levels of protective antioxidants that limit renal injury. Perhaps the finding by Yakulov et al. that urine glucose levels are lower in the Cxcl12 and Myc deficient mice is related to an effect on this nitric oxide pathway? Here, a more refined investigation into the timing of when the nitric oxide, Cxcl12, and Myc pathways are operative in IR-AKI is needed. Similarly, it will be necessary to have a good handle on how different cell types respond to these pathways. For instance, cells from the distal nephron are more resistant to ischemia during IR-AKI, whereas the proximal tubule has less capacity to convert from an oxidative to a glycolytic metabolism (23). With regards to non-tubular cells, Lemos et al. recently reported that a Myc-mediated switch to glycolysis in kidney stromal cells induces their proliferation and drives renal fibrosis (24). Lemos and colleagues took advantage of the human kidney organoid model to confirm their findings and this platform may be useful in the future to tease apart cell-specific responses and to determine if migratory repair processes can be visualized in mammalian tubules.
Despite many questions remaining, the Yakulov study has advanced our understanding of how the kidney tubule responds to injury and offers several new fruitful avenues to explore. Hopefully, these new research directions will lead to novel approaches to treat ischemic injury in the kidney and other organs.
Acknowledgements
We thank Professor Mark de Caestecker for critical reading and expert advice on AKI in the mouse model.
Funding: This work is supported by grants HRC(NZ) 17/425 and NIH-NIDDK(USA) RO1DK06940310 to AJ Davidson.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet 2013;382:170-9. [Crossref] [PubMed]
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007;18:1292-8. [Crossref] [PubMed]
- Yakulov TA, Todkar AP, Slanchev K, et al. CXCL12 and MYC control energy metabolism to support adaptive responses after kidney injury. Nat Commun 2018;9:3660. [Crossref] [PubMed]
- Drummond IA, Davidson AJ. Zebrafish kidney development. Methods Cell Biol 2016;134:391-429. [Crossref] [PubMed]
- Naylor RW, Dodd RC, Davidson AJ. Caudal migration and proliferation of renal progenitors regulates early nephron segment size in zebrafish. Sci Rep 2016;6:35647. [Crossref] [PubMed]
- Palmyre A, Lee J, Ryklin G, et al. Collective epithelial migration drives kidney repair after acute injury. PLoS One 2014;9:e101304. [Crossref] [PubMed]
- Vasilyev A, Liu Y, Mudumana S, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol 2009;7:e9. [Crossref] [PubMed]
- Vasilyev A, Liu Y, Hellman N, et al. Mechanical stretch and PI3K signaling link cell migration and proliferation to coordinate epithelial tubule morphogenesis in the zebrafish pronephros. PLoS One 2012;7:e39992. [Crossref] [PubMed]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009;457:495-9. [Crossref] [PubMed]
- Slanchev K, Pütz M, Schmitt A, et al. Nephrocystin-4 is required for pronephric duct-dependent cloaca formation in zebrafish. Hum Mol Genet 2011;20:3119-28. [Crossref] [PubMed]
- Donà E, Barry JD, Valentin G, et al. Directional tissue migration through a self-generated chemokine gradient. Nature 2013;503:285-9. [Crossref] [PubMed]
- Carroll PA, Freie BW, Mathsyaraja H, et al. The MYC transcription factor network: balancing metabolism, proliferation and oncogenesis. Front Med 2018;12:412-25. [Crossref] [PubMed]
- Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012;2:1303-53. [PubMed]
- Racker E, Resnick RJ, Feldman R. Glycolysis and methylaminoisobutyrate uptake in rat-1 cells transfected with ras or myc oncogenes. Proc Natl Acad Sci U S A 1985;82:3535-8. [Crossref] [PubMed]
- Braun M, Qorraj M, Büttner M, et al. CXCL12 promotes glycolytic reprogramming in acute myeloid leukemia cells via the CXCR4/mTOR axis. Leukemia 2016;30:1788-92. [Crossref] [PubMed]
- Stromski ME, Cooper K, Thulin G, et al. Chemical and functional correlates of postischemic renal ATP levels. Proc Natl Acad Sci U S A 1986;83:6142-5. [Crossref] [PubMed]
- Chiba T, Skrypnyk NI, Skvarca LB, et al. Retinoic Acid Signaling Coordinates Macrophage-Dependent Injury and Repair after AKI. J Am Soc Nephrol 2016;27:495-508. [Crossref] [PubMed]
- Huen SC, Cantley LG. Macrophages in Renal Injury and Repair. Annu Rev Physiol 2017;79:449-69. [Crossref] [PubMed]
- Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008;2:284-91. [Crossref] [PubMed]
- Oginuma M, Moncuquet P, Xiong F, et al. A Gradient of Glycolytic Activity Coordinates FGF and Wnt Signaling during Elongation of the Body Axis in Amniote Embryos. Dev Cell 2017;40:342-53.e10. [Crossref] [PubMed]
- Moriuchi M, Moriuchi H, Margolis DM, et al. USF/c-Myc enhances, while Yin-Yang 1 suppresses, the promoter activity of CXCR4, a coreceptor for HIV-1 entry. J Immunol 1999;162:5986-92. [PubMed]
- Zhou HL, Zhang R, Anand P, et al. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 2019;565:96-100. [Crossref] [PubMed]
- Bagnasco S, Good D, Balaban R, et al. Lactate production in isolated segments of the rat nephron. Am J Physiol 1985;248:F522-6. [PubMed]
- Lemos DR, McMurdo M, Karaca G, et al. Interleukin-1β Activates a MYC-Dependent Metabolic Switch in Kidney Stromal Cells Necessary for Progressive Tubulointerstitial Fibrosis. J Am Soc Nephrol 2018;29:1690-705. [Crossref] [PubMed]


