
Ultrasound patterns of pulmonary edema
Since its first description in 1967 (1), acute respiratory distress syndrome (ARDS) represents a well known major clinical problem in intensive care units (ICUs), carrying a high morbidity and mortality. Differentiating between hydrostatic or cardiogenic pulmonary edema (CPE) and ARDS is challenging, especially in the early stages of illness (2). This diagnostic task becomes more difficult in older patients, where a higher number of morbidities often coexists. Moreover, in septic patients with ARDS, the myocardium is dysfunctional due to systemic inflammatory activation and mitochondrial impairment, and sepsis related cardiomyopathy (3) is a recognized cause of left ventricular failure and of an increase in hydrostatic extravascular lung water and CPE.
The Berlin definition for ARDS (4) shows a better predictive power for mortality than the previous ARDS definition by the American-European Consensus Conference (AECC) (5), but it includes potential differential diagnostic inconsistencies, mainly related to the poor sensitivity and specificity of the chest radiographic criteria for distinguishing ARDS from CPE.
Nowadays, patients are presumed to have ARDS if they show respiratory distress which cannot be explained by findings of heart failure or fluid overload on a clinical basis and by using all the available data, because the pulmonary capillary wedge pressure measurement has been removed from this definition. Therefore, in current practice ARDS is usually differentiated from CPE by the clinical picture and by physical findings, and this distinction is often completed by the response to therapy.
The main characteristic of ARDS is the damage to the alveolar-capillary membrane which results in the accumulation of protein-rich fluid inside the alveolar septa and inside the alveoli (6). The histopathological features of ARDS follow three overlapping phases: an inflammatory phase with exudation of a protein rich fluid, a proliferative and cellular phase (often after only 72 hours) and a fibrogenic phase. The accumulation of perivascular and intra-alveolar fluid increases lung weight, whereas patients who develop cellular proliferation fibrosis and type 2 pneumocyte hyperplasia show an anatomical remodelling of the lung, reduction in pulmonary compliance (stiff lung), worsening in gas exchange and increased mortality.
Classical teaching states that capillary permeability edema is distinctive in chest X-rays and, mostly, in CTs. Its distribution is frequently peripheral, patchy and non-gravitational. Moreover, in ARDS the heart can be normal in size, pulmonary and intrathoracic blood volume is not increased, and Kerley B-lines and peribronchial/perivascular cuffing are rare (7).
In CPE, the first site of accumulation of extravascular lung water (a plasma ultrafiltrate) is in the loose connective tissue surrounding the blood vessels and bronchi. This space is continuous without interruptions with the interlobular septa and the subpleural tissue. The ongoing edema progressively expands and dilutes the extravascular fluid compartment around the vessels where the edema is taking place, creating an osmotic pressure gradient directed from the periphery to the center of the lung, where the osmotic pressure is higher. This effect facilitates the peripheral clearance of the fluid toward the center of the lungs (8), and represents a valid compensatory mechanism which prevents alveolar flooding.
What is more interesting from an ultrasonographic and diagnostic point of view, is that in CPE fluid expands the secondary interlobular septa in a deterministic fashion, which is mainly related to a pressure gradient. Therefore, the thickening of the individual septum is limited by its own physical capacitance and when it is maximally distended (interstitial tamponade) alveolar flooding occurs (9). On the contrary, in ARDS the inhomogeneous damage to the alveolo-capillary membrane generates an exudative edema. In this process, the pressure gradient between the intravascular and extravascular spaces is not primarily involved, but the exudation is mainly linked to the direct exposure of the alveolar and vascular cells to infective pathogens or toxic substances, and/or it is due to a general inflammatory cascade with release of cytokines and other mediators, producing diffuse panlobular damage. Consequently, the edema of ARDS is randomly distributed, anatomically patched, cellular and fibrogenic, and it tends to quickly become intra-alveolar, without a deterministic previous intraseptal stage (8-10).
Ultrasound is a valid instrument to detect an increase in the superficial density and air space distribution of the lung (11,12). Acute CPE and ARDS are diseases that increase the density of the superficial lung and the full/empty ratio of the subpleural lung tissue, but in different ways. Therefore, lung ultrasonography is generally considered a useful clinical tool among physicians. Many diagnostic ultrasound algorithms were recently developed to assist in the assessment of dyspnea, consolidations and interstitial lung diseases.
A characteristic ultrasound picture of the hyperdense, non-consolidated superficial lung is sonographic interstitial syndrome (SIS): the presence of multiple focal, patched or diffuse vertical artifacts (B-lines) fanning out from the lung-wall interface (13). White lung, characterized by a granular and mostly white texture which starts at the pleura line and ends at the bottom of the screen, is also an aspect of SIS for some authors (14).
The link between subpleural non-consolidative hyperdensities of the lung and SIS is related to the acoustic behaviour of the pleura that pathologically diverges from a normal specular reflector. In other words, SIS appears when the pleura is no longer a near-total reflector and a lot of B-Lines are generated from the pleural line erasing the specular A-line pattern (15).
The main characteristic of the diseases linked to SIS is the increase in the full-to-empty ratio of lung tissue immediately beneath the pleura and the altered spatial distribution of the residual air. In the presence of edema, ARDS, interstitial lung diseases, non-consolidative pneumonia and contusions, part of the lung volume which was originally occupied by air may be replaced with water, connective, cells, hyaline membrane or edematous tissue, ultimately creating acoustic traps for the US beam containing a medium that is physically (in terms of acoustic impedance) very different with respect to its surroundings (air). It is probable that, when insonated, these acoustic traps may behave like ultrasonic wave generators at specific frequencies (15,16), where the frequencies will depend on the content, size and shape of the trap. Therefore, the ultrasound imaging of SIS is an artefactual piece of information, whereas consolidations produce real anatomical images when the superficial lung is nearly completely (or completely) free of air.
The criteria for the ultrasound differential diagnosis between ARDS and CPE were proposed in 2008 (14), and in 2017 these criteria were reviewed (17) with particular attention being paid to the appearance, the genesis and the characteristics of SIS, in ARDS and in CPE, respectively.
Spared areas of a sonographically normal lung, along with areas with SIS, represent a sign of ARDS, while CPE does not show spared areas. Recently, Sekiguchi et al. confirmed this hypothesis showing that a higher “B-line ratio” (proportion of chest zones with positive B lines relative to all zones examined) was indicative of CPE (18).
During normal breathing, sonography can detect the “lung sliding”, i.e., the reciprocal movement of the lung and chest wall, when they are normally apposed and free from adhesions. While normal lung sliding is seen in subjects with CPE, it is impaired in patients with ARDS when the compliance of the lung is reduced (14).
Finally, in ARDS the progression from interstitial edema to the peripheral flooding and alveolar collapse produces lung consolidations with air bronchograms. Consolidations are unusual in CPE. Compared with CPE, the alveolar edema of ARDS usually has a more peripheral distribution, which can be easily seen with ultrasound, especially in the lowest areas of the lung (14,17).
In the study of Huang et al. (19), ultrasound images on consecutive subjects who had been admitted to the ICU with the clinical diagnosis of suspected ARDS were evaluated according to the recommendations by the International Consensus on Lung Ultrasound (13) and to the ARDS diagnostic criteria listed in the papers published in 2008 and 2017 (14,17).
In practical terms, the diagnosis of ARDS (33 patients) was based not only on the presence of SIS, but also on ancillary features, typical of ARDS, such as consolidations, abolition or impairment of lung sliding, spared areas of normal parenchyma and non-homogeneous distribution of B-Lines. 18 patients fell into the non ARDS group.
This study showed that lung ultrasound diagnostic results were consistent with CT results on days 1, 2 and 3 with the highest consistency on day 3. Sensitivity, specificity and AUROC using ultrasound for the diagnosis of ARDS were: 0.788, 0.778 and 0.783 on day 1; 0.909, 0.833 and 0.871 on day 2; 0.970, 0.833 and 0.902 on day 3.
While gravity dependent consolidations (a typical finding on a chest CT scan in ARDS) are easily recognized with ultrasound, SIS of ARDS and SIS of CPE are clearly different, especially when we observe an early hydrostatic edema, and hence deserve a supplementary analysis.
Our hypothesis considers SIS to be the very variable expression of the acoustic properties of a pleural surface, which is no longer a smooth specular reflector. Many acoustic events, which are linked to altered histologies in interstitial lung diseases and to pleural irregularities, develop in acoustic channels which are distributed along the pleural surface and produce many different types of B-lines. These phenomena are related to the intrinsic artefactual nature of the pulse echo imaging process, which is particularly evident when US meets high acoustic impedance mismatches, and to the presence of variously arranged superficial air spaces surrounding US permissive tissue (15) which ultimately give rise to acoustic traps of various sizes and shapes. Technically, a specific pulse activates specific native frequencies of an acoustic trap and the obtained vertical artefact assumes a consequent visual structure (16).
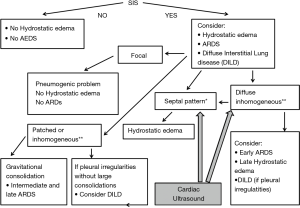
Thickened (but anatomically intact) secondary interlobular septa may act as acoustic traps where specific frequencies give rise to separated, uniform, bright and long B-lines, without spared areas (septal pattern, see Figures 1 and 2), especially in early CPE (Figure 1) (17). In ARDS subpleural random peri and intralobular distortion may explain the appearance of acoustically permissive irregular channels, different arrangements of B-lines, pleural irregularities, consolidations, and inhomogeneous isolated air spaces. Inhomogeneous edema, alveolar collapses and pulmonary fibrosis contribute to the development of the pleural irregularities that are seen in ultrasound and to the reduction of the pulmonary compliance and pleural sliding in ARDS (Figure 3) (9).
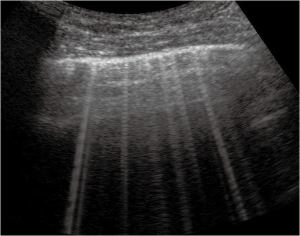
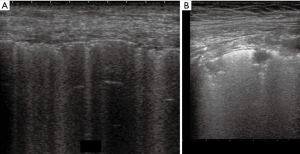
In other words, in ARDS an early complex tissue arrangement is at the basis of the very variable images that can be seen in sonography. On the contrary, the appearance of typical septal B-lines (shown in early CPE) is not usual in ARDS, when discrete septal thickening surrounded by anatomically normal air spaces is not present.
It is interesting to note that in CPE the septal thickening ceases and alveolar flooding occurs (20) when the secondary interlobular septa are maximally distended by the transudative fluid. Alveolar edema takes place with the all-or-none phenomenon (either fluid or air fills each single alveolus) (21). Flooding and alveolar instability, together with the collapse of peripheral airspace, generate many different kinds of acoustic channels and different artifacts with many arrangements. Therefore, pulmonary cardiogenic edema, at the later stages of the pathology, may lose the sonographic characteristic of septal discrete B-lines and tend more and more to resemble the edema of early ARDS without consolidations.
This can lead to diagnostic uncertainties when the clinician performs an ultrasound lung scan in a subject with respiratory distress. Moreover, other diagnostic difficulties arise when a patient with CPE is also affected by diffuse interstitial lung disease (with pleural irregularities and consolidations), or when subjects with ARDS develop some form of left heart failure. This is not a rare occurrence in older patients.
In all these cases, the qualitative analysis of the B-lines, does not yet allow clinicians to make a clear distinction, and the study of the cardiocirculatory function and/or the use of ex-adiuvantibus criteria are useful tools.
Echocardiography is a powerful instrument to detect and to monitor many cardiovascular and respiratory conditions (22). Since CPE develops through pulmonary capillary congestion, its diagnosis commonly relies on the detection of elevated left ventricular filling pressure (LVFP) in patients with systolic and diastolic left heart dysfunction (after the exclusion of mitral stenosis). Secondarily, echocardiography permits the diagnosis of underlying other heart diseases.
Elevated LVFP is diagnosed by the use of pulse-wave Doppler interrogation of mitral inflow (E and A waves) and tissue Doppler estimation of lateral and/or septal velocity of the mitralic annulus during early diastole (E' wave). Let Ev and Av be the E and A wave velocities, LVF is usually normal in patients with an impaired relaxation pattern Ev/Av <1 and Ev peak <50 cm/s. In subjects with restrictive pattern (Ev/Av ≥2 and E wave deceleration time <150 ms) LVFP is often increased. When Ev/Av ratios is between 1 and 2 other Doppler estimations are necessary to detect patients with increased LVFP.
The distinctive feature of systolic heart failure is a left ventricular ejection fraction <45–50%, whereas the diagnosis of heart failure with preserved left ventricular function relies on the presence of signs or specific symptoms of heart failure, LVEF >50% and no significant left ventricular enlargement (23).
In the study of Huang et al. (19) LVEF and stroke volume were estimated by echocardiography. In the studied population (fifty-one patients) if LVEF was ≤35% the subjects with respiratory distress were considered to have CPE and classified as non ARDS cases. If LVEF was between 35% and 50%, the clinical picture and lung ultrasound were considered for a differential diagnosis of ARDS and non ARDS. The performance of the differential diagnosis between ARDS and non ARDS subjects improved when both lung and cardiac ultrasound data were used. The sensitivity, specificity and AUROC were: 0.879, 0.889 and 0.924 on day 1; 0.939, 0.889 and 0.961 on day 2; 0.970, 0.833 and 0.956 on day 3.
In conclusion, lung ultrasound rapidly provides useful information in patients presenting with respiratory distress (24). The presence of SIS, consolidations and pleural irregularities confirms that the lung is more dense in its subpleural tissue, and that the normal pleural reflector is variably interrupted by acoustically permissible channels. The distinction between cardiogenic and pneumogenic pathologies relies on signs that are strictly related to the subpleural histology in terms of full (acoustically permissive tissue) and empty (residual air) (25-27).
Focal findings, consolidations, spared areas, pleural irregularities and impaired pleural sliding are typical signs of primitive lung involvement. SIS with bright, long, separated, modulated B-lines arising from a normal pleural line is strongly predictive of early CPE. Figure 2 illustrates an algorithmic approach used in our clinical practice.
In practical terms, therefore, the ultrasound picture of early CPE can be said to be characteristic, as is the ultrasound picture of ARDS in day 3, due to the presence of septal pattern in CPE and of inhomogeneous SIS and consolidations in ARDS, respectively. In late CPE, when alveolar flooding takes place, the sonographic picture tends to become confused with that of early ARDS, which in many cases, however, shows spared areas. The association of diffuse interstitial diseases and CPE is equally confounding.
These difficulties can be overcome with the use of cardiac ultrasound, and specifically with an estimate of the volume and function of the left ventricle and with an estimate of the left ventricular telediastolic pressure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome advances in diagnosis and treatment. JAMA 2018;319:698-710. [Crossref] [PubMed]
- Drosatos K, Lymperopoulos A, Kennel PJ, et al. Pathophysiology of sepsis related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep 2015;12:130-40. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med 1995;332:27-37. [Crossref] [PubMed]
- Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med 2005;353:2788-96. [Crossref] [PubMed]
- Milne ENC, Pistolesi M, Miniati M, et al. The radiologic distinction of cardiogenic and noncardiogenic edema. AJR Am J Roentgenol 1985;144:879-94. [Crossref] [PubMed]
- Elicker BM, Jones KT, Naeger DM, et al. Imaging of acute lung injury. Radiol Clin North Am 2016;54:1119-32. [Crossref] [PubMed]
- Summers RL, Amsterdam E. Pathophysiology of acute decompensated heart failure. Heart Fail Clin 2009;5:9-17. [Crossref] [PubMed]
- Soldati G, Smargiassi A, Inchingolo R, et al. Lung ultrasonography may provide an indirect estimation of lung porosity and airspace geometry. Respiration 2014;88:458-68. [Crossref] [PubMed]
- Soldati G, Inchingolo R, Smargiassi A, et al. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol. 2012;38:1169-79. [Crossref] [PubMed]
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577-91. [Crossref] [PubMed]
- Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008;29:6:16.
- Soldati G, Demi M, Inchingolo R, et al. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J Ultrasound Med 2016;35:2075-86. [Crossref] [PubMed]
- Demi L, van Hoeve W, van Sloun RJG, et al. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Sci Rep 2017;7:12746. [Crossref] [PubMed]
- Soldati G, Demi M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J Ultrasound 2017;20:91-6. [Crossref] [PubMed]
- Sekiguchi H, Schenck LA, Horie R, et al. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure. Chest 2015;148:912-8. [Crossref] [PubMed]
- Huang D, Ma H, Xiao Z, et al. Diagnostic value of cardiopulmonary ultrasound in elderly patients with acute respiratory distress syndrome. BMC Pulm Med 2018;18:136. [Crossref] [PubMed]
- Gluecker T, Capasso P, Schnyder P, et al. Clinical and radiologic features of pulmonary edema. Radiographics 1999;19:1507-31. [Crossref] [PubMed]
- Escolar JD, Escolar A. Lung hysteresis: a morphological view. Histol Histopathol. 2004;19:159-66. [PubMed]
- Vignon P, Repessè X, Vieillard Baron A, et al. Critical care ultrasonography in acute respiratory failure. Critical Care 2016;20:228. [Crossref] [PubMed]
- Lancellotti P, Price S, Edvardsen T, et al. The use of echocardiography in acute cardiovasculare care: Recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2015;4:3-5. [Crossref] [PubMed]
- Smargiassi A, Inchingolo R, Soldati G, et al. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med 2013;8:55. [Crossref] [PubMed]
- Soldati G, Demi M, Smargiassi A, et al. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev Respir Med 2019;13:163-72. [Crossref] [PubMed]
- Demi L, Demi M, Smargiassi A, et al. Ultrasonography in lung pathologies: new perspectives. Multidiscip Respir Med 2014;9:27. [Crossref] [PubMed]
- Zanforlin A, Giannuzzi R, Nardini S, et al. The role of chest ultrasonography in the management of respiratory diseases: document I. Multidiscip Respir Med 2013;8:54. [Crossref] [PubMed]


