Study on the digitized and quantified evaluating method for the super information cluster of traditional Chinese medicine ultraviolet spectral fingerprints
Introduction
Nowadays, the quality testing about traditional Chinese medicine (TCM) is always by High Performance Liquid Chromatography (HPLC) method with ultraviolet detector. Although the ultraviolet spectrum analysis technology has an important application in quantitative determination, the features of only single information contained and ordinary characteristics results in its poor practical usage in the study of TCM fingerprint information. Meanwhile the extracts of TCM or their preparation certainly contain dozens or even hundreds of components, so the qualitative and quantitative information acquired at single or several wavelengths is obviously not comprehensive and not overall (1,2). Consequently, ultraviolet absorption spectra in the region 190-400 nm in a dilute solution can cover the superposed information of chemical composition, that is to say, ultraviolet fingerprint (UVFP) can be served as a simple and accurate quantitative technology for TCM identification (3,4). In addition, the information of unsaturated chemical bonds and conjugate system of the total contents in TCM extract can be detected or determined by UV spectrum analysis. It is just this fact that provides the basis for testing the overall chemical contents in TCM accurately by UVFP. In this paper, the evaluating method of the Super Information Cluster of Ultraviolet Spectrum Fingerprints was further developed according to the theories of UVFP Index, Information Index, Fluctuation Index, Information Fluctuation Index and quantified Ultraviolet fingerprint method (QUFM). GTs, the most popular herbal drug (HD) used extensively in at least 130 countries with $5 billion worldwide sales per year (5), can increase peripheral and cerebral blood flow, treat dementia and decrease mental vitality at old age, tinnitus, Alzheimer’s disease and depression (6-8). Numerous studies had reported to detect the contents of flavone glycosides and terpene lactones which were much inadequate to represent its real therapeutic effect (9-11). Furthermore, increasing interest in chemical fingerprint especially HPLC fingerprint (HPLC-FP) analysis can be observed, however, complex data processing, longer analysis time, and pollution of organic solvent limited its utility in manufacturer’s accompanying inspection.
Therefore, in this study, we developed the UVFP as a fast, accurate, and effective method to reveal the complex system of GT based on the change of the multidimensional super information cluster obtained from the 211 UV data points from 190-400 nm. An effective, practical, simple and accurate method came into being to display the overall quantitative characteristics of the TCM with the feature of complex giant system by using UVFP.
Theory for digitized super information of UVFP (12,13)
UVFP index theory
Fingerprint point n
Considering each peak point in UVFP as fingerprint point, each fingerprint point represents the total absorbency of various chemical components (only thoroughly saturated chemical component cannot be effectively detected). Using all peak points of UVFP to quantitatively evaluate the overall quality of TCM possesses the advantages of abundantly, accurately and briefly quantitative information that was a very important innovation for TCM quality control.
Fingerprint separation ratio (β)
Fingerprint separation ratio was defined to reflect the peak point numbers and purity of monochromatic light seen in Eq. [1], where Δλ, d (nm), n, λ1 and λ2 is the adjacent wavelength interval of a continuous spectrum, slit width, fingerprint points, the starting and ending wavelength of scanning, respectively. The bigger fingerprint point number and the much purer of monochromatic light are, the larger β, which is usually one.
Fingerprint frequency ρ
Fingerprint frequency ρ is termed as the number of peak points obtained in unit wavelength, seen in Eq. [2], which usually is 1 or 2.
Fingerprint total signal intensity (LR)
Fingerprint total signal intensity (LR) is defined as the total sum of each fingerprint point absorbance, seen in Eq. [3], which represents the total signal overlap intensity of all fingerprint points, where Ai (Ti) is the absorbance or transmittance under the ith wavelength.
Fingerprint AUC
Fingerprint AUC is the integral area under curve of an ultraviolet spectrum that is performed by Simpson's integral method, so it is another index to reveal the total contents of TCM chemicals. When ρ is equal to one, AUC is equal to LR.
Leveling coefficient of peak signal γ
Leveling coefficient of peak signals γ is termed as the intersection angle cosine of and
to reveal how uniform are the fingerprint signals, seen in Eq. [4]. The closer to one for γ, the poorer characteristics of a profile. Likewise, the geometric arithmetic ratio δ is defined as the ratio of the geometric mean of absorbances to the arithmetic mean of absorbances, respectively, seen Eq. [7], where
and A0 are the arithmetic mean of absorbances and geometric mean of absorbances, separately, seen Eq. [5,6] that can also reveal how uniform are the peak signals.
Fingerprint space occupying ratio η
Fingerprint space occupying ratio η is defined as the percentage of AUC accounted for the largest spectrum area that is the wavelength range multiples the biggest value of absorbances, seen Eq. [8].
Apparent injection mass Q (mg)
Q is an apparent mass of raw materials or their preparations to be prepared sample solution, of which extracts have been loaded to be analyzed during UVFP process, where its unit is 1 mg.
Apparent absorption coefficient E
Apparent absorption coefficient E is defined as the absorbance of extractum of 1 mg raw materials or preparations at a certain wavelength, where the maximum of Emax is usually selected to express the UVFP characteristics, seen Eq. [9].
[1] |
[2] |
[3] |
[4] |
[5] |
[6] |
[7] |
[8] |
[9] |
UVFP index F
Taking the signal response, leveling coefficient, separation ratio and effective information into consideration, UVFP index F is defined to directly indicate both the fingerprint information amount and the signal intensity and also the uniformity, seen Eq. [10].
UVFP standard index Fr(q)
Considering the influence of different injection mass, UVFP standard index Fr(q) is F corrected to Q=1 mg for further expressing the mass information of a sample, seen Eq. [11].
Fixed wavelength range index Fr(λ)
Besides the fingerprint information amount and the signal intensity and uniformity, Fr(λ) is termed as Eq. [12] that can reveal the spectrum efficiency of the whole profile at a fixed wavelength range.
The relative index Fr
Fr is defined as a comprehensive relative index that shows the relative information acquired at the condition of a fixed wavelength range and per unit injection mass, seen Eq. [13]. If the number of fingerprint points is 1,000, and Ai is equal to 1.0, meanwhile γ, ρ, and β is 1, respectively, then Fr is less than 100.
[10] |
[11] |
[12] |
[13] |
Information amount theory
Peak point entropy Si
According to Shannon entropy, the fingerprint peak point entropy Si may be defined as Eq. [14,15], where pi is the normalization value (AT) of each fingerprint point absorbance (Ai).
Total fingerprint entropy S
Total fingerprint entropy S is the sum of each peak points entropy Si, seen Eq. [16]. The greater the S the more information reflected by it.
Fingerprint information amount index I
I is defined as the total sum of the natural logarithm of absorbance peak Ai multipled by the entropy Si, leveling coefficient of peak signal γ and the effective entropy, seen Eq. [17]. So it can comprehensively show how high the signal responses are, leveling coefficient of peak signals and the information amount of the fingerprint profiles.
Standard information amount index Ir(q)
Ir(q) is the corrected I by the apparent loading mass, accounting for 1 mg of the crude drug or preparation denoted as Eq. [18]. Beside the signal responses, leveling coefficient of the peak signals and the information amount of the profiles, it can also represent the apparent loading mass of the raw materials or their preparations that will be extracted for sample solution.
Fixed wavelength range information amount index Ir(λ)
In order to express the spectrum efficiency, I is corrected by the fixed range of wavelength to get Ir(λ) that is shown in Eq. [19], which refers to I multiplied by 200 and divided by the difference of the beginning scan wavelength λ1 and the ending one λ2 to depict the information contained whether more or less than that acquired at 200 nm wavelength.
Relative information amount index Ir
Summing the above two factors, i.e., the apparent loading mass and the spectral efficiency, Ir is defined to display how about the relative information of chemical constituents in samples, seen Eq. [20]. Not only the signal responses, leveling coefficient of the peak signals, and the information amount, but also the loading mass of the raw materials or their preparations and the spectral efficiency of the profile can be thoroughly described by this parameter.
Inverse ratio of two indexes ω
In order to compare the difference between F and I, the inverse ratio of the two indexes ω was shown in Eq. [21], for which is often less than 1.0. The uniformity gets better as the ω value gets bigger.
[14] |
[15] |
[16] |
[17] |
[18] |
[19] |
[20] |
[21] |
The number of arrest points and the central tendency theory
In order to further explore a UVFP fluctuation, the number of arrest points Nsp, the fingerprint range R and the fingerprint range ratio Rstd have been proposed.
The number of arrest points Nsp
Nsp is termed as the number of the first derivatives being zero that are namely the extreme points on the curve, which obviously reveals the fluctuation of a fingerprint profile. The bigger the number is, the heavier the fluctuation. However, smoothing a profile can cause the number down.
Fingerprint range R
R is the difference between maximum and minimum point on a UV curve, which can characterize the variation of the fingerprint distribution and that of the dispersion degree.
Fingerprint range ratio Rstd
Rstd is defined as the ratio of maximum absorbance and fingerprint range R, seen Eq. [22]. It is another parameter to characterize the variation of the fingerprint distribution and that of the dispersion degree as well as R.
Median ratio m
What’s more, the median ratio m is defined as the ratio of the average absorbance to the maximum absorbance, which depicts how closer the fingerprint points are to the central tendency. When m does exceed 0.5 to denote the number of the higher absorbance points is bigger, neither the case nor the number of those is big. If m is equal to 1, in which the uniformity is the best, but the characteristic of UVFP is very poor, seen Eq. [23].
[22] |
[23] |
Fluctuation amount index theory
Fluctuation amount index AF
AF is termed as such an index that takes the fingerprint leveling coefficient, the number of arrest points, the fingerprint range ratio, the effective information entropy and the total responses all into consideration, described as Eq. [24]. It can tell us how about the total signal responses, the effective amount of information, the uniformity and the fluctuation of a UV curve. Actually AF is the sum of each absorbance common logarithm when γ=1, Nsp=0, m=1 and Rstd=∞. The better leveling coefficient, the higher responses and the larger information amount will contribute to a bigger value of AF.
Standard fluctuation amount index AFr(q)
AFr(q) is the corrected AFr after testing extract from 1 mg of the raw materials or their preparation denoted as Eq. [25]. A lot of information including the total signal response, the effective amount of information, the uniformity, the fluctuation of a UV curve and the apparent loading mass can be comprehensively expressed by this index.
Fixed wavelength range fluctuation amount index AFr(λ)
AFr(λ) is termed as the corrected AFr by the fixed range of 200 nm, seen Eq. [26]. The greater fluctuation positively correlates the smaller range of wavelength. AFr(λ) synthesizes the spectral wavelength efficiency besides the information of AFr.
Relative fluctuation amount index AFr
The above two parameters are simultaneously taken into calculation: the AFr is defined as Eq. [27]. It is such an index that manages to disclose not only the leveling coefficient, the number of arrest points, the fingerprint range ratio, the effective information entropy and the total response but also the apparent loading amount and the spectral efficiency.
[24] |
[25] |
[26] |
[27] |
Information fluctuation amount index theory of UVFP
Information fluctuation index AI
In order to establish an index combined the total amount of information with the fluctuation degree, AI is put forth as Eq. [28]. It is a comprehensive index that can simultaneously indicate how about the fingerprint leveling coefficient, the number of arrest points, the fingerprint range ratio, the effective space information entropy and the total response. If the ideal conditions are met γ=1, Nsp=0 and Rstd→∞, then . A bigger value of AF usually means the larger volatility of the effective spatial information.
Standard information fluctuation index AIr(q)
Assumed the most adequate 1 mg of Q, AI is corrected by the apparent loading amount to be called the standard information fluctuation index AIr(q) denoted as Eq. [29], which refers to the practical AI as Q is corrected as equal to 1.0 mg.
Fixed wavelength range information fluctuation amount index AIr(λ)
Considering the spectral efficiency, AIr(λ) is termed as the corrected index of AI seen Eq. [30], which refers to AI multiplied by 200 and divided by the difference of the beginning scan wavelength λ1 and the ending one λ2 to depict information contained whether more or less than that acquired in 200 nm.
Relative information fluctuation amount index AIr
AIr defined as Eq. [31] is introduced for correcting the apparent loading mass and the spectral efficiency for the same purpose as AFr. It is such an index that manages to comprehensively disclose how high are the leveling coefficient, the number of arrest points, the fingerprint range ratio, the effective space information entropy and the total signal response as well as the apparent loading amount and the spectral efficiency of the profile.
Inverse ratio of fluctuation indexes aω
The inverse ratio of fluctuation aω is applied to compare the difference between AF and AI just as ω, seen Eq. [32]. The uniformity is positively correlated with the value of aω.
[28] |
[29] |
[30] |
[31] |
[32] |
Quantified UV fingerprint method (QUFM)
Although the digitized parameter criteria of the supper information characteristics for HD or TCM-UVFP has many abundant information, that cannot directly provide the detailed quality control information. Thereof the accurately qualitative and quantitative measurements of all chemicals have not been effectively performed. Based on the complexity science principle, we proposed a powerful method called quantified UV fingerprint method (QUFM) to effectively assess the quality level of GT. QUFM, taking each spectral point as the calculating unit, is a method combining the macro qualitative similarity Sm in Eq. [33] with the macro quantitative similarity Pm in Eq. [34] and the relative deviation coefficient α in Eq. [35] to simultaneously monitor or identify TCM and HD authentic quality level, in which xi and yi are the peak point absorbance of a sample and the reference UVFP, respectively, meanwhile mRFP and mi are the loading mass. The method combined Sm with Pm and α to determine or identify TCM quality level is called QUFM, in which the TCM and HD quality is divided into 8 grades in terms of QUFM criteria, listed in Table 1.
[33] |
[34] |
[35] |

Full Table
Principle for the development of UVFP
Flowing injection analysis (FIA) method
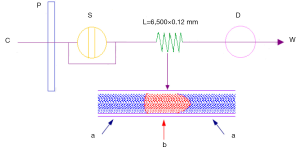
Ultraviolet spectrum possesses the fingerprinting characteristics of the different chemical composition system for its specially recognizing the chemical information of π→π*, n→π* and n→σ*. Therefore, this feature makes the UVFP much possible to perform the quality control of TCM as well as its preparation. To avoid the shortcomings of traditional ultraviolet analysis, FIA method shown in Figure 1 was employed in high performance liquid chromatography (HPLC) system. FIA method, which used a packless column instead of the reversed phase column and detected the ultraviolet signal by DAD, had the advantages of high speed, stability and accurate reproduction.
Establishment of reference UVFP
For fingerprinting similarity analysis, one of the most common and easiest applying tools is to establish the reference fingerprint that is a crucial step. As for establishing the reference of UVFP, two following approaches can be employed. One way is calculating the whole ultraviolet spectral fingerprint points of more than 10 batches of the representative TCM raw materials or the extract solution of patent medicines by the averaging method. Another one is determining the famous-region drug or standard preparations by continuously for 6 times to develop the mean mode of them. Obviously, the mean or median fingerprint of the data set is usually taken for it is more feasible and more likely to achieve the accompanying quantitative analysis. Generally speaking, the reference UVFP can be used as the qualitative and quantitative standard after eliminating the system errors from the different instruments.
Types and characteristics of UVFP
UVFP for the extract approaches that directly exert an effect upon the stability and reproducibility essentially into four categories: UVFP of water-soluble ingredients, UVFP of liposoluble components, UVFP of whole components and UVFP of effective and characteristic groups. As one of the modern analytical means for the fingerprint testing, the UVFP has the following advantages: (I) rapid analysis (analysis time less than 1 min); (II) high stability and repeatability; (III) abundant quantitative information (detect in a wide range of wavelength at less 190-400 nm); (IV) accurately quantitative analysis; (V) significant digital characteristics. Therefore, even despite the single qualitative assay, the UVFP is more comprehensive and more precise than HPLC-UV in reflection of the whole information of TCM chemical ingredients.
Experimental
Apparatus and reagents
The analysis was performed on an Agilent 1100 HPLC series (Hewlett Packard, CA), equipped with a DAD detector, low pressure quaternary pumps, an online degasser and an autosampler. A Sarturius-BS110S analytic scale (Saiduolisi scale company limited, Beijing, China) was used during the analysis.
Methanol (purchased from Yuwang Industry Limited Company, Shandong, China), was HPLC grade. The other reagents were all analytical grade.
The 14 batches of GTs were bought from shenyang drugstores to specify as following. S1 (091103) and S12 (110602) were from the pharmaceutical factory A; S2 (20100901) and S11 (20110601) were from factory B; The others, S3 (20110101), S4 (110101), S5 (110202), S6 (110303), S7 (110406), S8 (1104218), S9 (1105015), S10 (1105251), S13 (110809) and S14 (20110901) were all produced in different factories, respectively. The names of manufacturers had been removed to maintain confidentiality.
Preparation of the sample solution
Ten tablets of GTs were accurately weighed to get the average weight for each one. A quantity equivalent to two tablets in powdered states was weighed and extracted with 20 mL methanol in an ultrasonic water bath for 20 min. The extracted solution was filtered and then diluted to 25 mL in a flask with methanol. The solution was centrifuged at 3,500 rpm for 10 min, and then the supernatant obtained was filtered through a 0.45 µm Millipore filter before analysis.
Experimental conditions
The unseparated chromatograms in UVFP were gained on an Agilent polytetrafluoro-ethylene (PTFE) tube (6,500 mm × 0.12 mm) with the column temperature of 30.00 (±0.15) °C. The mobile phase was methanol at a flow rate of 0.5 mL/min and with an injection volume of 0.5 µL. Data acquisition was at the wavelength of 190-400 nm (DAD), with interval of 1 nm and slit width of 1 nm. Therefore 211 data points were gained in every UV spectrum.
Data analysis
All data acquired were processed by a ChemStation workstation (Agilent technology Inc). Similarity analysis of UV based on QUFM was performed on software 3.0 of Digitized Evaluation System for Super Information Characteristics of TCM UV Fingerprints (software Certificate No: 0462756 China) invented by Prof. Guo-Xiang Sun, etc.
Results and discussion
Experiment condition selection and method validation
Selection of flow rate
In this study, the flow rate (Fc) of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 mL/min were investigated as loading 0.50 µL sample solution and the ultraviolet spectra at 190-400 nm were recorded. It suggested that a lower Fc resulted in a higher AUC with the regression equation of y=151942-7642.5x, r=–0.9589. Finally, 0.5 mL/min was selected because there was no great difference between the ultraviolet spectra in the range of 0.5 to 1.0 mL/min shown in Figure 2A and the maximum absorbance of UVFP was rightly appropriate.
Selection of injection amount
The injection volume shown in Figure 2B of 0.20, 0.40, 0.50, 0.60 and 0.70 µL were loading into the column with the flow rate of 0.5 mL/min and the ultraviolet spectra at 190-400 nm were recorded. Obviously a linear correlation (y=11616x+7429.6, r=1.00) can be observed between AUC and the apparent injection amount in the range of 3.41 to 11.93 µg. Collecting spectrum at the peak point after deducting the nearest baseline based on its 3D spectrum, loading 0.50 µL had an advisable absorbance in the range of 600-800 mAU, in which 0.50 µL was eventually selected.
System suitability test
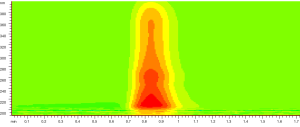
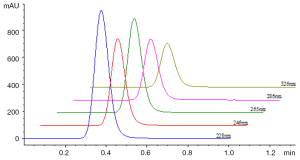
0.5 µL of sample solution was loaded and the contour map of absorbance of sample in UV spectral region from 190-400 nm was recorded as Figure 3. The unseparated chromatograms at 228, 246, 265, 286 and 326 nm selected on the basis of the contour map were recorded and compared shown in Figure 4. Theoretical plate number of this system was no less than 54 as the analysis time for 1 min.
Methodology validation
All tests below were carried out on the sample solution prepared as described in Section 4.2. The injection precision was determined by replicate HPLC injections of the same sample solution 6 times per day. Precision of sample stability was determined with measurements from a single sample solution stored at room temperature for 0, 1, 2, 4, 6 and 8 h. The retention time (RT) and peak area (PA) were recorded for estimating the precision and stability and the results were as following—precision: the relative standard deviations (RSD) of RT and PA were found not to exceed 0.40% and 1.70%, respectively; sample stability—below 0.40% and 1.40%, respectively. Thus, all results indicated that the quality of the studied samples and the FIA coupled with HPLC-DAD measurements were relatively stable and under control.
Development of the UVFP and analysis (14-16)
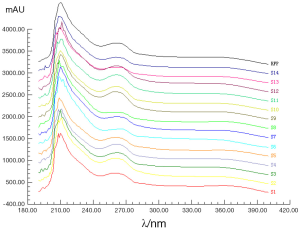
Under the present approach, the UV fingerprint of each sample was recorded. The UV absorption spectral fingerprints of 14 batches of GT samples from 190 to 400 nm were revealed in Figure 5. The variation between the 14 samples was significant especially from 205 to 280 nm. Two obvious broad peaks at 265 and 360 nm were the spectral feature flavonoids or their glycosides components in GTs possibly, such as kaempferol, quercetin and rutin. While majority of components with chemical bonds π→π*, n→π* in structure may contribute to the strong absorption at 210 nm, like terpene lactones components in GTs.
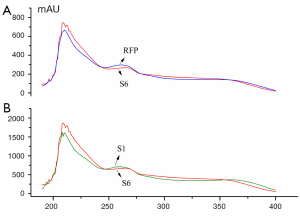
The UV spectra of 14 batch of GT samples and their reference fingerprint (RFP) obtained by the averaging method were converted into the file layout of CSV respectively and imported into the above software 3.0. Similarity parameters Sm, Pm and α and final quality grades were presented in Table 2, which were all based on each point absorbance in every wavelength within 190-400 nm. It was investigated through all samples that were almost similar with a low relative standard deviation (RSD) of 0.12%, the qualitative similarity Sm of S6 is the lowest which was mainly due to the variance of absorbance in the region of 240-400 nm shown in Figure 6A. Obviously, the absorbance of S6 was lower than the RFP in the range of 240-275 and 350-400 nm, however it was higer in the range of 275-350 nm. Even qualitative similarity Sm was not characteristic for the comparison of UVFP, it was proved that subtle difference can be distinguished. For the quantitative similarity Pm, the great difference can be observed with high RSD of 29.86% from batch to batch which makes a obvious distinction between samples directly. As the results shown in Table 2, sample S2 and S11 from the manufactory B were proved to be accordant in quality, while sample S1 and S12 from the same source got the inconsistent results. It was conceivable that being active at keeping relatively stable raw material or technology in one company does well to the batch-to-batch consistency of the final products. Generally speaking, all batches were qualified (Grade ≤3) except S8 for its too higher contents which also necessarily might harm the patient’s health and safety or lead to a series of problems to the therapeutic effect caused by overdosage.

Full Table
Evaluate GTs by the super information cluster of ultraviolet spectum fingerprints
The spectral data of 14 batches of GT samples was imported into the above software 3.0. Enormous information of the UVFP contained potentially was presented in Table 3. Part of results were described and analyzed as following: (I) fingerprint separation ratio (β) and fingerprint frequency (ρ) are all one which meet the requirement of fingerprints points numbers and purity degree of monochromatic light; (II) fingerprint total signal intensity LR=100,122-126,720, fingerprint AUC=99,991-122,749, arithmetic mean absorbance
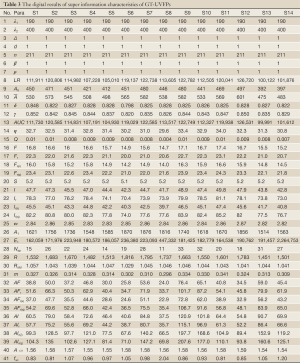
Full Table
Conclusions
An accessible and feasible digitized and quantified evaluating method for super information cluster of traditional Chinese medicine UVFP was successfully established and applied to explore the potential information of the UVFP. In this method, 46 parameters were together to explore the information of ultraviolet spectra which can scarcely been observed by the visual examination. The UVFP analysis achieved its fast testing by the FIA method with high stability, precision and accessiblity in practice. Based on the above theory, 14 batches of GTs were finally evaluated as the different quality grades. It was suggested that all batches were qualified (Grade ≤3) except S8 whose quality grade was inferior for its too high contents which also necessarily can lead to a series of influences to the therapeutic effect. Our results have demonstrated that employing multi-dimensional digital and quantitative UVFP identification to reveal the characteristics of whole chemical contents is a practical, powerful, reliable and feasible method which can also be comprehensively performed on the quality control of the diverse TCM concerning the accompanying monitoring in manufacturers.
Acknowledgements
This work was supported by the Important Research Plan “Study on the Scientific Action Circumtances Based on the Internet” of National Natural Science Foundation of China (No. 90612002).
Disclosure: The authors declare no conflict of interest.
References
- Lucio-Gutiérrez JR, Coello J, Maspoch S. Enhanced chromatographic fingerprinting of herb materials by multi-wavelength selection and chemometrics. Anal Chim Acta 2012;710:40-9. [PubMed]
- Yan SK, Xin WF, Luo GA, et al. An approach to develop two-dimensional fingerprint for the quality control of Qingkailing injection by high-performance liquid chromatography with diode array detection. J Chromatogr A 2005;1090:90-7. [PubMed]
- Devi Datt Joshi. eds. Herbal Drugs and Fingerprints Evidenced Based Herbal Drug. Berlin, Germany: Spriner, 2012.
- Ni LJ, Zhang LG, Hou J, et al. A strategy for evaluating antipyretic efficacy of Chinese herbal medicines based on UV spectra fingerprints. J Ethnopharmacol 2009;124:79-86. [PubMed]
- van Beek TA, Montoro P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J Chromatogr A 2009;1216:2002-32. [PubMed]
- Wu Y, Li S, Cui W, et al. Ginkgo biloba extract improves coronary blood flow in healthy elderly adults: role of endothelium-dependent vasodilation. Phytomedicine 2008;15:164-9. [PubMed]
- Abdel-Wahab BA, Abd El-Aziz SM. Ginkgo biloba protects against intermittent hypoxia-induced memory deficits and hippocampal DNA damage in rats. Phytomedicine 2012;19:444-50. [PubMed]
- Rickard NS, Kowadlo N, Gibbs ME. Effect of the Ginkgo biloba extract, EGb 761, on memory formation in day-old chicks. Pharmacol Biochem Behav 2001;69:351-8. [PubMed]
- Ch.P. 2010;2010:1079-82.
- Gray DE, Upton R, Chandra A, et al. Quantitative analysis of flavonol glycosides in Ginkgo biloba: a comparison of two analytical methods. Phytochem Anal 2006;17:56-62. [PubMed]
- Li G, Zeng X, Xie Y, et al. Pharmacokinetic properties of isorhamnetin, kaempferol and quercetin after oral gavage of total flavones of Hippophae rhamnoides L. in rats using a UPLC-MS method. Fitoterapia 2012;83:182-91. [PubMed]
- Sun GX, Hu YS, Bi KS. Evaluating the quality of niuhuangjiedu tablets by the systematic quantified fingerprint method. Yao Xue Xue Bao 2009;44:401-5. [PubMed]
- Sun GX, Yang TT, Che L. Quality identification of Liuwei Dihuang Wan by UV- IR spectrum systematic quantified fingerprint. Central South Pharmacy 2010;8:766-71.
- Sun GX, Zhi XZ, Zhang CL, et al. Digitized evaluation system for super-information characteristics of chromatographic fingerprints in traditional Chinese medicine. Central South Pharmacy 2007;5:549-55.
- Sun G, Bi K. Effect of fingerprintology of traditional Chinese medicine (TCM) in the innovative development of TCM. Se Pu 2008;26:172-9. [PubMed]
- Sun GX, Hou ZF, Bi YM, et al. The digital criterion of the potential information characteristics of the traditional Chinese medicine chromatographic fingerprints. Yao Xue Xue Bao 2006;41:857-62. [PubMed]