Is severe re-expansion pulmonary edema still a lethal complication of closed thoracostomy or thoracic surgery?
Introduction
Re-expansion pulmonary edema (REPE) is a complication that may occur after inserting a chest tube. Lung injury develops due to increasing endovascular permeability, which is caused by rapid expansion of the collapsed lung during insertion of a chest tube (1). The incidence of REPE is thought to be low, especially as REPE causing clinically significant respiratory failure is very rare (2-4). Some reviews have evaluated case reports, but there is no clinical research report about the progress and treatment of REPE. We retrospectively analyzed the common clinical characteristics, clinical progress, and treatment outcomes of patients with REPE and respiratory failure.
Methods
We retrospectively reviewed the clinical features, treatment, and outcomes of eight patients with REPE who required ventilator care from March 2004 to March 2018. Inclusion criteria were patients who underwent intubation for severe hypoxia due to pulmonary edema caused by insertion of a chest tube or thoracic surgery. Other causes of pulmonary edema were excluded. Intubation was performed in patients who had severe dyspnea and did not maintain saturation over 95%, even with oxygen mask treatment of ≥10 L/min.
Treatment strategy
Patients with severe dyspnea and who did not maintain saturation >95% even with oxygen mask treatment of ≥10 L/min were intubated. The current principle of patient respiratory therapy is to start mechanical ventilation at a FiO2 value of 1.0, in 15–20 mmHg pressure control mode, and with positive end-expiratory pressure (PEEP) of 5–7 mmHg. If PaO2 is maintained above 100 mmHg and PCO2 is maintained below 50 mmHg, FiO2 should be reduced as soon as possible considering the extent of REPE improvement and hemodynamic stability. Tidal volume should not exceed 8 mL/kg and peak pressure should not exceed 30 mmHg. Prior to 2011, 8 mL/kg volume control ventilation and low PEEP (5–7 mmHg) was used, but the same pressure control method is now being implemented. However, the first two cases in this study were treated using 10 mg/kg volume-controlled ventilation with a high PEEP (10 mmHg). Changes in ventilator mode reflected the trend in ventilator therapy at the time of treatment.
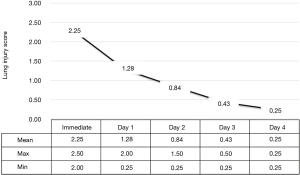
We used the PaO2/FiO2 ratio and the lung injury score (LIS) to assess the extent of lung injury and evaluate improvement in REPE. The LIS scoring system has four components: a chest X-ray score, a hypoxemia score, a PEEP score, and a respiratory system compliance score. The final value is obtained by dividing the aggregate sum by the number of components used. We calculated the LIS at the time of immediate intubation, and every day until extubation. When the final value of the LIS was <0.5 and patients were weaned off the ventilator. Additionally, hypovolemic hemodynamic instability due to leakage of edematous fluid was resolved by allocating the proper colloid volume replacement after maintenance of blood pressure using a catecholaminergic drug.
The study protocol was approved by the Institutional Review Board of Pusan national university Yangsan hospital and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived.
Results
Six men and two women were included in this study, with a mean age of 41.9±15.6 years (range, 17–64 years) (Table 1). The causes were pneumothorax (n=5), malignant effusion (n=2), and diaphragm eventuation (n=1). All REPE was caused by rapid lung expansion. The causes of rapid expansion were coughing (n=4), positive pressure ventilation during general anesthesia (n=2), rapid pleural fluid drainage (n=1) and suction (n=1). The mean time from chest tube insertion to intubation was 90.8±48.6 min (range, 40–180 min) in six cases, i.e., excluding two general anesthesia cases. There were 2 cases in which more than 120 min had passed before intubation; in both of those cases, intubation was required at 60 and 70 min after chest tube insertion but intubation was delayed due to the circumstances of our hospital.

Full table
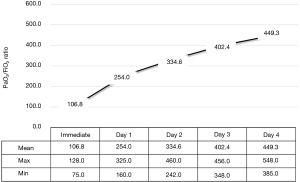
The mean PaO2/FiO2 ratio of the patients immediately after intubation was 106.5±20.2 (range, 75–128), indicating severe hypoxia in all cases. However, when the LIS was analyzed, all cases showed moderated acute lung injury (2≤ LIS ≤2.5). This is because the contralateral lung without REPE was generally compliant and chest PA findings showed no increase in opacity in the contralateral lung. The severity of hypoxia and lung injury in patients using a mechanical ventilator decreased markedly. On the first day of treatment, most of the patients had improved hypoxia, and by day 3 most improved and were extubated. Extubation was possible in all patients on day 4 of treatment (Figures 1,2).
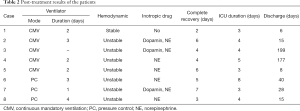
The results of the REPE treatment are summarized in Table 2. The volume-control ventilator mode was used mainly before 2010, and the pressure-control mode was applied thereafter. These changes in ventilator mode may be due to changes in trends in acute respiratory distress syndrome therapy. Initial hemodynamic instability occurred in all patients. All patients were treated with inotropic agents, except for one 24-year-old man. The mean duration of ventilator use was 2.5±0.8 days (range, 1–4 days), and the mean duration of inotropic use was 1.1±0.7 days (range, 0–2 days). The mean duration of the intensive care unit (ICU) stay was 4.4±1.5 days (range, 3–8 days). Most patients recovered rapidly within one week (Figure 3A,B). The mean length of hospital stay was 61.0±70.0 days (range, 6–190 days). The difference in length of hospital stay was due to individual patient’s disease rather than the effect of REPE. No deaths were recorded during the study period and there were no cases of permanent complications due to REPE.
Full table
Discussion
Understanding the pathophysiology and characteristics of REPE is necessary to determine the optimal treatment. Chronic lung collapse causes thickening of the pulmonary capillary endothelium and basement membrane, resulting in decreased flexibility of blood vessels. Under these conditions, a collapsed lung may suddenly expand after insertion of a chest tube, resulting in increased blood flow and, in turn, sudden expansion of inflexible blood vessels, resulting in vascular injury and increased pulmonary blood permeability. The sudden blood supply to the collapsed lung produces oxygen-derived free radicals, resulting in lung damage similar to ischemic reperfusion lung injury. This damage causes infiltration of leukocytes in the lungs and ultimately induces pulmonary insufficiency. All cases had causes for sudden expansion. A mistake during chest tube insertion and ventilator management caused the rapid lung expansion.
REPE symptoms gradually worse necessitating use of a ventilator to improve breathing difficulties. However, use of a ventilator does not improve oxygenation as expected (5). Because lungs with pulmonary edema show lower compliance and the contralateral lung is normally compliant, positive ventilation can cause excessive expansion of normal lungs when the edematous lung is relatively non-ventilated. These phenomena may result in decreased pulmonary artery blood flow to the normal lung and increased edema in the normal lung. Therefore, a ventilator is not helpful for patients with REPE. In the early stages of severe REPE, ventilation perfusion mismatch, which develops due to mechanical positive pressure ventilation, may render it difficult to maintain oxygenation and hemodynamics; therefore, mechanical positive pressure ventilation may be of little utility for resolving hypoxemia (6). Overcoming this challenge is the most important element of successful REPE treatment.
Fortunately, the critical period is not long and the patient’s condition rapidly improves when the initial hypoxemia is solved. In the patients in this study, vital signs stabilized within 48 hours after use of a ventilator and oxygenation improved within 24 hours of respirator use (1). This observation indicates that survival probability increases if the 1–2-day period in which death is most likely to occur is overcome; most such patients in this study were discharged without sequelae.
It is necessary to maintain oxygenation in a patient without lung damage until lung function is restored, to overcome the initial high-risk period. PEEP therapy is the first-choice method for adequate oxygenation. Application of positive pressure inhibits plasma leakage from the capillary arteries and interstitial tissue to the alveoli, and facilitates oxygenation by maintaining a high concentration of oxygen in the alveoli (7). However, the more PEEP and positive pressure ventilation increase, the more a normal lung expands (8). Because the expanded lungs compress, pulmonary resistance increases in the pulmonary artery, the supply of blood to the overinflated normal lungs is relatively reduced, and pulmonary blood flow increases in the edematous lung (which has relatively low pulmonary resistance). Furthermore, the ventilation perfusion mismatch (V/Q mismatch) increases and the oxygen supply decreases. Therefore, it is necessary to avoid high PEEP and over-inflation of the normal lung to overcome the initial V/Q mismatch after ventilator care. In addition, as well as ventilating a high tidal volume, PEEP can also cause blood flow mismatch.
Therefore, we avoided high PEEP and performed low rather than high tidal volume ventilation. We conclude that ventilation using high-concentration O2 gas is the only way to resolve low saturation due to use of a low PEEP and low tidal volume, even though use of high O2 concentrations may lead to lung toxicity (8). We lowered the oxygen concentration as soon as possible, to reduce the likelihood of lung damage, if we judged the oxygen exchange capacity of the lung had improved.
As shown by our results, most of the patients improved within 24 hours, showing that the period of high oxygen was insufficiently long to cause lung damage. Thus, we conclude that lung toxicity due to high oxygen therapy is not dangerous when treating REPE. FiO2 decreased within 24 hours of ventilator care in most cases. Increasing the oxygen concentration rather than the PEEP and tidal volume proved a more effective method for treating REPE.
Because there was no original article that reported the mortality of REPE with severe respiratory failure, the accurate mortality is not known. Only we can predict the mortality by analyzing the case reports. Mahfood et al. reported a mortality rate of 20% in the 53 cases they reviewed. These results likely represent an inflated mortality rate secondary to the inherent selection bias present in case series. Considering our result, the actual mortality would be lower than 20%. However, if severe REPE occurs, the possibility of mortality can’t be ruled out.
Fortunately, in our study, all patients survived with conventional ventilator support and appropriate critical care. However, the conventional technique alone cannot be used to treat all cases of severe REPE. If the patient is difficult to treat using conventional treatments, such as our regimen, an alternative treatment is needed as a second option. The first alternative to consider is differential ventilation of the bilateral lungs through double lumen intubation; this is an ideal method to solve a V/Q mismatch by applying low PEEP and low tidal ventilation to the normal lung, and high PEEP to the edematous lung (3,6,9). However, two ventilators are needed and there are few reported cases; thus, we cannot be sure regarding the efficacy of this treatment. The second option is extracorporeal membrane oxygenation (ECMO). Veno-venous ECMO is the most effective treatment for patients with a high mortality risk, where the V/Q mismatch could be resolved by an increase in blood saturation. As the rate of use of ECMO is higher in patients with respiratory insufficiency, the application of ECMO for treating REPE will increase (10). However, given its high cost, ECMO would not be appropriate as a first-line treatment for patients with REPE who are difficult to maintain on a ventilator.
In conclusion, REPE requiring ventilator care can be a lethal disease. However, as shown by the results of this study, recent developments in ICU care have dramatically decreased mortality. In particular, mortality does not occur if V/Q mismatch is properly treated during the early period of ventilator care. We recommend that low PEEP, low tidal volume, and high O2 ventilator care represents the most appropriate treatment for REPE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board of Pusan national university Yangsan hospital and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived.
References
- Mahfood S, Hix WR, Aaron BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg 1988;45:340-5. [Crossref] [PubMed]
- Ault MJ, Rosen BT, Scher J, et al. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-32. [Crossref] [PubMed]
- Sakellaridis T, Panagiotou I, Arsenoglou A, et al. Re-expansion pulmonary edema in a patient with total pneumothorax: a hazardous outcome. Gen Thorac Cardiovasc Surg 2012;60:614-7. [Crossref] [PubMed]
- Kira S. Reexpansion pulmonary edema: review of pediatric cases. Paediatr Anaesth 2014;24:249-56. [Crossref] [PubMed]
- Sohara Y. Reexpansion pulmonary edema. Ann Thorac Cardiovasc Surg 2008;14:205-9. [PubMed]
- Achar SK, Chaudhuri S, Krishna H, et al. Re-expansion pulmonary oedema - differential lung ventilation comes to the rescue. Indian J Anaesth 2014;58:330-3. [Crossref] [PubMed]
- Mora Carpio AL, Mora JI. Positive End-Expiratory Pressure (PEEP). StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC.; 2018.
- Thomson L, Paton J. Oxygen toxicity. Paediatr Respir Rev 2014;15:120-3. [Crossref] [PubMed]
- Papakonstantinou DK, Gatzioufas ZI, Tzegas GI, et al. Unilateral pulmonary oedema due to lung re-expansion following pleurocentesis for spontaneous pneumothorax. The role of non-invasive continuous positive airway pressure ventilation. Int J Cardiol 2007;114:398-400. [Crossref] [PubMed]
- Kim HC, Suh KH, Lee YC. Severe Bilateral Re-Expansion Pulmonary Edema Successfully Managed With Extracorporeal Membrane Oxygenation After Robot-Assisted Mitral Valve Repair Surgery. J Cardiothorac Vasc Anesth 2016;30:1038-41. [Crossref] [PubMed]