
Ceritinib-related interstitial lung disease improving after treatment cessation without recurrence under either crizotinib or brigatinib: a case report
Introduction
Lung cancer is the first cause of mortality by cancer worldwide (1), with an estimated 1.8 million new cases (12.9% of all cases) and 1.6 million deaths (19.4% of the total) worldwide in 2012 (1). Non-small cell lung cancer (NSCLC) accounts for around 85% of cases, with adenocarcinoma being currently the most common histological subtype (38.5%) (2). Since the beginning of the 21st century, several driver mutations have been detected in non-squamous cell carcinoma (NSqCC), first including adenocarcinoma and undifferentiated large-cell carcinoma epidermal growth factor receptor (EGFR) activating mutations, then followed by anaplastic lymphoma kinase (ALK) translocations (3). These oncogenic driver mutations like EFGR activating mutations and ALK rearrangements are more common in never smokers (4). Indeed, 44% EGFR activating mutations and 12% ALK rearrangements are found in NSqCC of never smokers, as compared to 11% EGFR activating mutations and 5% ALK rearrangements in overall NSqCC (4).
These driver mutations or rearrangements that are targetable have led to a new revolution in cancer treatment with significantly better patient outcomes, namely response rates of about 70%, progression free survival (PFS) between 9 to 11 months, as well as median survival time between 16.5 to 27 months (4,5) as compared to a maximum of 12 months under platinum-based chemotherapy (6). In 2014, crizotinib, a first-generation ALK inhibitor (ALKi), proved its superiority in comparison with pemetrexed-plus-platinum chemotherapy as first-line agent for ALK-positive lung cancer patients (7). However, over time, most patients develop resistance to crizotinib, which new-generation ALKi can overcome. Ceritinib, a second-generation ALKi, demonstrated significant clinical efficacy in crizotinib-refractory ALK-rearranged NSCLC patients (8). Brigatinib, another second-generation ALKi, has proven its clinical efficacy in patients who progressed under crizotinib therapy, especially in those with brain metastases (9).
Case presentation
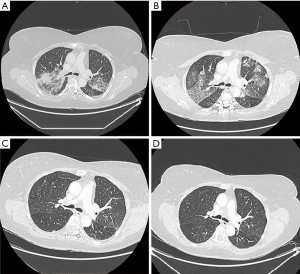
In December 2014, an ALK-positive adenocarcinoma of the left lower pulmonary lobe staged cT4N3M1b (7th edition) was diagnosed in a 53-year-old woman, never smoker and without previous medical history. The present case had distant metastases (pulmonary, pericardial, bone, and adrenal lesions). The patient was included in a clinical trial with ceritinib, with treatment initiated in February 2015. After 7 months of treatment, the patient developed progressive dyspnea with a dry cough leading to hospitalization in September 2015. A thoracic computed tomography (CT)-scan revealed alveolar condensations and ground-glass opacities of the two upper lobes, along with a thickening of the inter-lobular septa of both apexes (Figure 1A). Upon arterial blood gas analysis, there was a PaO2 of 73.2 mmHg, PaCO2 of 35.1 mmHg, and pH of 7.46. Blood analysis displayed a leucocyte count at 10.88×109/L (9.14×109/L neutrophils, 0.95×109/L lymphocytes, and 0.11×109/L eosinophils), hemoglobin level at 13.5 g/dL, and platelet count at 354×109/L. Serum C-reactive protein level was elevated at 119.3 mg/L. A bronchial fibroscopy with bronchioloalveolar lavage (BAL) was performed, with 110 mL fluid instilled and 40 mL fluid collected. BAL fluid analysis revealed 1,440,000/mL leucocytes (90% lymphocytes with a TCD4/TCD8 ratio of 1.4, 5% neutrophils, 1% eosinophils, and 4% macrophages). Bacteriological and parasitological cultures were negative. No other etiology of interstitial lung disease (ILD) was found, namely no concomitant medications, no infection, and no progressive disease. Treatment with ceritinib was immediately discontinued. After one week without ceritinib administration, along with high-dose corticosteroid therapy consisting of 1.5 mg/kg daily for 5 days, respiratory symptoms had completely regressed with normalization of blood biology, especially a serum C-reactive protein level decreased at 6.1 mg/L. The thoracic CT-scan showed a significant regression of alveolar and interstitial images (Figure 1B). In November 2015, crizotinib was initiated without relapse of ILD (Figure 1C). After 1-year crizotinib treatment, the patient developed multiple brain metastases. Thus, brigatinib was introduced in January 2017. Fourteen months after treatment initiation, there were no signs of relapse of ILD (Figure 1D).
Discussion
In clinical trials with ceritinib, drug-related ILD was reported to occur with a frequency of 1.1% (10). This serious adverse event has been described with all ALKi, independent of their generation, and especially with brigatinib (7%) (10). Our case described ceritinib-related ILD without recurring under two different ALKi. Our patient had no known ILD risk factors (mainly previous ILD or interstitial pulmonary fibrosis) (11). Doménech et al. reported a similar case of a patient with ALK-rearranged NSCLC who developed crizotinib-induced ILD without ILD relapse with brigatinib (12). On the other hand, Pellegrino et al. reported a case of a patient who developed brigatinib-induced ILD and lung toxicity relapse with ceritinib (10). Management of these drug-related pulmonary adverse events secondary has proven to be a challenge in current practice in thoracic oncology. Generally speaking, after eliminating all other etiological diagnoses of ILD, mainly infection, lymphangitic carcinomatosis, and other concomitant medication, the concerned drug must be stopped and corticosteroids be administered for a few weeks, whilst gradually tapering their dosages (10,13). Whereas the evolution after drug discontinuation is favorable in the majority of cases, it may be fatal in about 9% of cases (10). Ceritinib-related ILD was reversible upon discontinuation of ceritinib and steroid treatment in our patient. The introduction of another ALKi treatment did not induce ILD relapse. This observation suggests that ALK-positive NSCLC patients treated by ALKi therapy who develop ILD could continue treatment, though with a different ALKi. Several hypotheses have been proposed to identify the mechanism responsible for ALKi-related ILD in NSCLC patients. Créquit et al. suggested that crizotinib-related ILD could be considered as a hypersensitivity pneumonitis. Indeed, the long delay before respiratory symptoms occur, which is accounted for by a phase of drug sensitization, along with symptom resolution after treatment cessation and high-dose corticosteroid administration, as well as the relapse after drug re-introduction, which was however not attempted in our case, are all in favor of a hypersensitivity mechanism (14). Moreover, BAL fluid in our patient revealed a majority of lymphocytes with a low CD4/CD8 ratio, which likewise suggested a hypersensitivity reaction. These authors proposed a second theory, given that in their view, crizotinib-related ILD could result from an immune antitumor response. Indeed, the authors reported the case of five patients who developed ILD occurring a long time after crizotinib introduction, all patients exhibiting a favorable tumor response. In addition, PFS proved to be longer in these patients versus patients without lung toxicity (19.9 versus 6.2 months, respectively) (14). Moreover, while all ALKi may be at the origin of ILD, its incidence is apparently not identical among the different ALKi treatments. It should, however, be stressed that other studies are necessary to fully explain the pathophysiological mechanism of ALKi-related ILD. To conclude, this case suggests that the occurrence of an ALKi-related ILD should not be considered to be an absolute contraindication to use another ALK tyrosine kinase inhibitor. Indeed, while all ALKi may be responsible for ILD, lung toxicity relapse does not systematically occur under a different ALKi therapy. However, re-challenge of another ALKi to patients with previous history of ALKi-related ILD and with ILD risk factors must be approved in a collegial decision. Moreover, patients must be informed about the potential risk of ILD-relapse in order to obtain their consent. Physicians should exercise caution when re-challenging of another anti-cancer therapy to patients with previous ALKi-related ILD, especially patients with ILD risk factors. Indeed, those patients could develop severe or lethal ILD. We propose that regularly follow-up be implemented for patients who developed an ILD over the past and were then switched to another ALK tyrosine kinase inhibitor, and this in an effort to promptly detect pulmonary toxicity recurrence and discontinue treatment as necessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: B Mennecier and E Quoix were investigators of clinical trials regarding crizotinib, ceritinib, brigatinib and lorlatinib. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Jain RK, Chen H. Spotlight on brigatinib and its potential in the treatment of patients with metastatic ALK-positive non-small cell lung cancer who are resistant or intolerant to crizotinib. Lung Cancer (Auckl) 2017;8:169-77. [Crossref] [PubMed]
- Pellegrino B, Facchinetti F, Bordi P, et al. Lung Toxicity in Non-Small-Cell Lung Cancer Patients Exposed to ALK Inhibitors: Report of a Peculiar Case and Systematic Review of the Literature. Clin Lung Cancer 2018;19:e151-61. [Crossref] [PubMed]
- Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. [Crossref] [PubMed]
- Doménech M, Jové M, Aso S, et al. Successful treatment with brigatinib in a patient with ALK-rearranged lung adenocarcinoma who developed crizotinib-induced interstitial lung disease. Lung Cancer 2018;119:99-102. [Crossref] [PubMed]
- Tamiya A, Okamoto I, Miyazaki M, et al. Severe acute interstitial lung disease after crizotinib therapy in a patient with EML4-ALK-positive non-small-cell lung cancer. J Clin Oncol 2013;31:e15-7. [Crossref] [PubMed]
- Créquit P, Wislez M, Fleury Feith J, et al. Crizotinib Associated with Ground-Glass Opacity Predominant Pattern Interstitial Lung Disease: A Retrospective Observational Cohort Study with a Systematic Literature Review. J Thorac Oncol 2015;10:1148-55. [Crossref] [PubMed]


