THERANOSTICS—clinical aimshots in surgical warfare against well-differentiated neuroendocrine neoplasms
Specificity of interaction between therapeutic agents and their targets is the key to success in medicine. It was Paul Ehrlich, more than 110 years ago, who postulated (probably for the very first time) in his famous side-chain theory that there is a specific interaction between cells and pathogens. Although not completely accurate, the side-chain theory gave rise to discovery of receptors capable of binding specifically, one of the bases of immunology. A later expansion lead to the concept of magic bullets or “therapia magna sterilisans” which indicated medication neutralizing pathogens in concentrations harmless to the human body and resulted in preparation Ehrlich 606, a trivalent arsenic compound against treponema pallidum, called Salvarsan (1).
An evolution of principles proposed by Paul Ehrlich laid the foundations of THERANOSTICS, which indicates a combination of therapeutics and diagnostics applying the same vector. It implicates diagnostic testing to find specific treatment avenues so as to ensure personalized treatment with minimal unwanted effects. Molecular therapy of cancer is an example of THERANOSTICS. For example, for the treatment of breast cancer using HER-2 antibodies, molecular profiling of cancer tissues for over expression of HER-2 receptors is mandatory. Nuclear medicine has promoted THERANOSTICS as sequence of molecular imaging and molecular therapy using radionuclides to select patients suitable for therapy. Early examples are using radioactive iodine for thyroid cancer. For neuroendocrine tumors, THERANOSTICS has realized its potential by targeting somatostatin receptors for both molecular imaging and molecular therapy in dedicated centers worldwide.
Neuroendocrine cancer may develop from virtually any organ originating from dispersed neuroendocrine cells of the diffuse neuroendocrine system. These tumors may be well or moderately differentiated with a less aggressive biological behaviour or rapidly growing poorly differentiated neuroendocrine carcinomas with a poor prognosis. The majority of well and moderately differentiated neuroendocrine tumors express G-protein coupled receptors for somatostatin in higher concentration than physiological expression in endocrine organs such as hypothalamus or thyroid. Somatostatin receptors (SSTRs) occur in 5 subtypes; In neuroendocrine cancer mostly SSTRs 1 and 2A are over expressed. Since somatostatin inhibits secretion by endocrine cells, it is therefore suitable for treatment of hormonal excess caused by functionally active neuroendocrine neoplasias. Stable somatostatin analogs were developed to circumvent short half-life of native somatostatin-14 and somatostatin-28. The cyclic octapeptides octreotide and lanreotide are the most commonly used somatostatin analogs available for a 4-weekly depot injection. These somatostatin analogs are suitable for therapy of functionally active neuroendocrine neoplasias and may also delay tumor progression in well-differentiated neuroendocrine neoplasias of small bowel. Somatostatin analogs, when used as anti-proliferative agents, have a low rate of remission, but only minor side effects (2).
SSTRs were first visualized by autoradiography 1984 by Reubi et al. in vitro and five years later in vivo by Krenning et al. using initially 123I-labelled [Tyr3] octreotide, which was later replaced by better suited [111In-DTPA0] octreotide, due to lower non-specific uptake. [111In-DTPA0] octreotide has become commercially available for somatostatin receptor scintigraphy (Octreoscan®, Covidien) and is the most widely used imaging technique for neuroendocrine neoplasias. Internationally accepted protocols for the performance of [111In-DTPA0] octreotide somatostatin receptor scintigraphy have been adopted by the Society of Nuclear Medicine, the European Association of Nuclear Medicine and the European Neuroendocrine tumor Society (3).
Endocrine und non-endocrine organs like thyroid, pituitary, adrenals, liver, spleen and kidneys are physiologically visualized by [111In-DTPA0] octreotide somatostatin receptor scintigraphy. Sensitivity for the detection of neuroendocrine neoplasias reaches 80% to 100% for neuroendocrine neoplasias of small bowel and 60% to 90% for pancreatic neuroendocrine neoplasias. Detection of neuroendocrine neoplasias by [111In-DTPA0] octreotide somatostatin receptor scintigraphy is mostly dependent upon over-expression of SSTR 2A.
Somatostatin receptor scintigraphy may also be performed with metastable Technetium-99 [99mTc] coupled to somatostatin analogs and some advantages have been reported. However, [111In-DTPA0] octreotide somatostatin receptor scintigraphy is still considered to be the international gold standard for the imaging of neuroendocrine neoplasias (3,4).
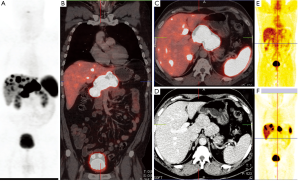
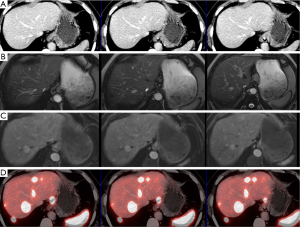
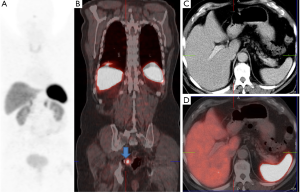
A significant step forward to increased sensitivity, improved spatial resolution, shorter imaging times, better anatomical localisation and lower radiation exposure was coupling positron emitters to somatostatin analogs for SSTR PET/CT (Figures 1,2). 68Gallium (68Ga) has been shown to be a nearly ideal positron emitter since it can be easily generated by an in-house 68Germanium generator and coupled to somatostatin analoges using different chelators. These positron emitting somatostatin analoges are DOTA peptides coupled to 68Gallium and include 68Ga-DOTA-TOC {[DOTA0, Tyr3]-octreotide}, 68Ga-DOTA-NOC {[DOTA0, 1-Nal3]-octreotide} and 68Ga-DOTA-TATE {[DOTA0, Tyr3, Thr8]-octreotide}. Minor differences exist between binding capabilities of 68Gallium DOTA peptides, which are not relevant in clinical practice. These 68Gallium coupled somatostatin analoges concentrate in neuroendocrine tumors very rapidly (80% within 30 minutes) and—those molecules not bound to tumor-are eliminated by renal clearance nearly immediately. Contrast is excellent since 68Gallium coupled somatostatin analoges depict low background radiation. Imaging is possible 30 to 180 minutes (ideal 60 to 90 minutes p.i.) after injection and radiation exposure to the patient is less than one-half of [111In-DTPA0] octreotide somatostatin receptor scintigraphy (3,4).
Tumor cells often depend upon uptake of glucose for metabolism (Warburg effect) and PET with 18F-fluorodeoxyglucose [18F-FDG] is suitable for poorly differentiated neuroendocrine neoplasias, but is not sensitive for well and moderately differentiated neuroendocrine tumors. Thus, 18F-FDG PET/CT may detect dedifferentiated rapidly proliferating neuroendocrine tumors or secondary tumors of non-neuroendocrine origin. Other PET/CT modalities for neuroendocrine tumors include 18F-DOPA and 11C-5-HTP but are less standardized (and more difficult to produce) than 68Gallium DOTA somatostatin receptor PET/CT (4).
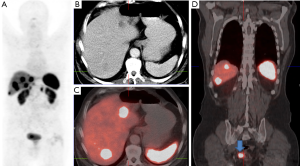
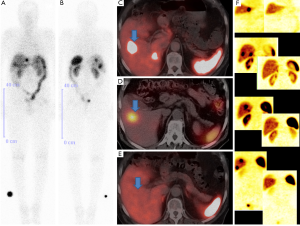
Somatostatin analogues marked with radionuclides may not only be used for imaging of somatostatin receptor expressing tumors but also for therapy known as peptide receptor radionuclide therapy (PRRT) by chelating beta- and gamma-emitters to the same peptide and chelator backbone as for imaging thus combining nuclear diagnostic and therapy (Figures 3-5). Initially, Krenning et al. first used 111Indium in high doses for peptide receptor radionuclide therapy in patients selected by high somatostatin receptor expression with [111In-DTPA0] octreotide somatostatin receptor scintigraphy. The results, however, were disappointing regarding response rates whereas the selected high activities resulted in high bone marrow and renal radiation dose yielding high numbers of unwanted effects (3).
To circumvent high background radiation, Yttrium-90 was chelated with DOTA to octreotide [90Y-DOTA0, Tyr3] octreotide [90Y-DOTATOC]. 90Yttrium is a pure beta-emitter with a half-life of 2.7 days, a therapeutic range of 12 mm and energy of 935 KeV. An initial study by Otte et al. from the Basel group examined 29 patients with intra-patient dose escalation protocols in 4 or more cycles with a cumulative dose of 6,120±1,347 MBq/m2. Of the 29 patients, 20 were stable at the end of the observation period, 2 had a partial remission, 4 a minor remission and three were progressive. Renal and hematological side effects were observed in 5/29 patients which was related to cumulative dose >7,400 MBq/m2 and absence of renal protective infusions (5). A follow-up study with prospective design included 39 patients with progressive neuroendocrine tumors of gastroenteropancreatic and bronchial origin. 4 cycles of 7.4 GBq/m2 were given at 6 weeks interval. Objective response rate according to WHO criteria was 23% (2/39 complete remission; 7/39 partial remission; 27/39 stable disease; 3/39 progressive disease). Side effects were grade 3 or 4 lymphocytopenia in 23%, grade 3 anemia in 3% and grade 2 renal insufficiency in 3% (6).
The European Institute of Oncology in Milan (Italy) performed a number of studies with 90Y-DOTATOC and reported an overall response rate of partial remission and complete remission in 27% of 256 patients, which were progressive at start of therapy in 80% of cases. Cumulative activity was 7.4 to 21.3 GBq applied in 3 or more courses. The maximal tolerated dose was calculated as 5.18 GBq per cycle since reversible hematological toxicitiy was observed in 43% of patients and no acute or chronic renal toxicity (7).
A prospective multi-center phase I trial (dose-escalation) with 90Y-DOTATOC was given to 58 patients with gastro-entero-pancreatic tumors either as 14.8 GBq/m2 in 4 cycles or as a single dose of up to 9.3 GBq/m2. Dosage was limited by estimated kidney dose of 27 Gy. In this study, renal protection was performed with amino-acid infusion. Of 58 included patients, 55 could be evaluated and these had mostly stable disease (29/55), 5/55 had partial remission, 7/55 minor remission and 13/55 progressive disease. Karnofsky status or symptoms were improved in more than half of the patients (21/36). Median overall survival was 36.7 months. Extent of disease and progression before therapy were negative predictors of overall survival (8).
Another prospective multi-center study was performed in 90 patients with neuroendocrine neoplasias of the gastro-entero-pancreatic system treated with a fixed dose of three cylces of 4.4 GBq 90Y-DOTATOC [90Y-edotreotide; Onalta®]. Median progression-free survival was 16.3 months and overall survival 26.9 months (9). Most patients experienced stable disease (70%) and 4% partial remission. Patients with symptomatic improvement had longer progression-free survival than patients without. Grade 3 and 4 renal failure occurred in one patient each. The largest study with 90Y-DOTATOC was recently reported by the Basel group and consisted of 1,109 patients which were treated with 3.7 GBq/m2 unless disease progression or permanent toxicity (2,472 cycles, median 2, range 1-10). Median overall survival of all patients after diagnosis was 94.3 months during a median follow up of 23 months and a death rate of 44.3%. Response rate was 34.1% including minor, partial and complete response rates. Overall survival was associated with morphological, biochemical and clinical response to therapy as shown by overall survival of patients with disease control compared to progressive patients (3.8 versus 1.4 years after start of treatment). Also high tumor uptake led to higher survival than low uptake. The strength of that study was meticulous analysis of side effects. Of all patients, 12.8 experienced grade 3 and 4 hematological toxicities and 9.2% grade 4 and 5 permanent renal toxicity, which is an impairment of high dose therapy with 90Y-DOTATOC despite survival advantage of responding patients (10). As such, contemporary protocols aimed to avoid renal and hematological toxicities by modifying affinity of radioligands and thus radiation exposure of bone marrow and kidneys.
As such octreotide was modified by replacing the C-terminal threoninol by threonine [DOTA0, Tyr3] octreotate (DOTATATE) increasing the affinity for somatostatin receptor subtype 2. A study with 90Y-DOTATATE in 75 patients led to high rate of partial remission and stable disease with similar side effects as 90Y-DOTATOC (11). In addition, lanreotide, another somatostatin analogue was coupled to 90Yttrium (DOTA-LAN) with increased affinity to somatostatin receptor subtype 5 and has been tested in the MAURITIUS trial in Europe (12). A trial with 154 patients revealed stable disease in 41% and tumor regression in 14%. However, despite its effectiveness, the clinical usage of 90Yttrium DOTA-LAN has been discontinued.
In search for radionuclides with improved tumor to kidney ratios, the beta- and gamma-emitting radionuclide 177Lutetium was tested, which has a half-life of 6.7 days, a therapeutic range of 2 mm and an energy of 133 keV of beta rays and 208 keV of gamma rays allowing for therapeutic dosimetry and scans. Chelated to [DOTA0, Tyr3] octreotate (DOTATATE), 177Lutetium-DOTATATE [177Lu-DOTATATE] demonstrated high tumor uptake and low renal radiation and was shown to be effective in early clinical trials (13). The trial was continued and 310 patients with neuroendocrine neoplasias were treated by the Rotterdam group with regularly 4 cycles with treatment intervals of 6-10 weeks and a total administered activity of 27.8-29.6 GBq (14). Results were promising; median overall survival from start of therapy was 46 months (128 months from initial diagnosis) and median progression-free survival was 31 months. When patients were progressive under therapy, median overall survival was 11 months whereas it was not reached in patients with stable disease and any type of remission. Median progression-free survival was 40 months in patients without progression as a treatment outcome. Response rates were encouraging: objective response rates as a combination of complete remission, partial remission and minor remission was 46%. Stable disease was achieved in 35% and 20% were progressing. Response rate was higher in functioning and non-functioning pancreatic neuroendocrine tumours than in neuroendocrine neoplasias of small bowel. Other prognostic factors were tumor uptake on the OctreoScan and a Karnofsky score of higher than 70%. Therapy with 177Lu-DOTATATE appears to spare kidney function compared to 90Y-DOTATOC, however, rates of hematological side effects are higher using this therapeutic regime (14). This landmark trial demonstrated advantage of overall survival of several years compared to historical controls with little side effects inaugurating peptide receptor radionuclide therapy as one of the most efficient therapies for patients with somatostatin receptor expressing neuroendocrine neoplasias. These results could be corrobated in a recently published prospective phase I/II trial from the Milano group with 177Lu-DOTATATE in 51 patients. Here rate of complete and partial remission was 32.6% and median progression free survival 36 months (15).
Further studies strived to maximize response and decrease side effects of peptide receptor radionuclide therapy. One approach, the BAD BERKA peptide receptor radionuclide therapy protocol combines lower radiation with longer therapeutic intervals with more frequent treatment cycles (up to nine) under stringent control of renal and hematologic function. Furthermore, this protocol utilizes both radionuclides, 90Ytttrium and 177Lutetium for combinatory (TANDEM) or sequential therapy (DUO). Other recent attempts employ different modes of application of the radionuclides such as intra-arterial delivery or use additional radiosensitizers such as capecitabine.
One of the drawbacks of peptide receptor radionuclide therapy is lack of commercially available and registered products. As such, delivery of peptide receptor radionuclide therapy has been focused on dedicated centers worldwide applying their self-generated products which did not allow generation of comparable clinical trials until lately. Due to continued efforts by E. Krenning and D. Kwekkeboom and many others from the Rotterdam group, the NETTER-1 trial started recently recruiting patients. In this prospective and randomized trial, patients with neuroendocrine neoplasias of small bowel and progressive disease under treatment with somatostatin analoges are randomized of 4 cycles of 177LuDOTATATE or high dose somatostatin analoge treatment. This trial will be the first step establishing a principle of peptide receptor radionuclide therapy as the therapeutic arm of THERANOSTICS to scores of patients with neuroendocrine neoplasias awaiting effective treatments worldwide.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Winau F, Westphal O, Winau R. Paul Ehrlich--in search of the magic bullet. Microbes Infect 2004;6:786-9. [PubMed]
- Hörsch D, Grabowski P, Schneider CP, et al. Current treatment options for neuroendocrine tumors. Drugs Today (Barc) 2011;47:773-86. [PubMed]
- Teunissen JJ, Kwekkeboom DJ, Valkema R, et al. Nuclear medicine techniques for the imaging and treatment of neuroendocrine tumours. Endocr Relat Cancer 2011;18:S27-51. [PubMed]
- Rufini V, Baum RP, Castaldi P, et al. Role of PET/CT in the functional imaging of endocrine pancreatic tumors. Abdom Imaging 2012;37:1004-20. [PubMed]
- Otte A, Herrmann R, Heppeler A, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999;26:1439-47. [PubMed]
- Waldherr C, Pless M, Maecke HR, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med 2002;43:610-6. [PubMed]
- Chinol M, Bodei L, Cremonesi M, et al. Receptor-mediated radiotherapy with Y-DOTA-DPhe-Tyr-octreotide: the experience of the European Institute of Oncology Group. Semin Nucl Med 2002;32:141-7. [PubMed]
- Valkema R, Pauwels S, Kvols LK, et al. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3] octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 2006;36:147-56. [PubMed]
- Bushnell DL Jr, O’Dorisio TM, O’Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol 2010;28:1652-9. [PubMed]
- Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23. [PubMed]
- Baum RP, Söldner J, Schmücking M, et al. Intravenous and intra-arterial peptide receptor radionuclide therapy (PRRT) using Y-90-DOTA-Tyr3-octreotate (Y-90-DOTA-TATE) in patients with metastatic neuroendocrine tumors. Eur J Nuc Med 2004;31 (Suppl 2): S238.
- Virgolini I, Britton K, Buscombe J, et al. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med 2002;32:148-55. [PubMed]
- Kwekkeboom DJ, Bakker WH, Kam BL, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [(177)Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging 2003;30:417-22. [PubMed]
- Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124-30. [PubMed]
- Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 2011;38:2125-35. [PubMed]


