
Meningeal lymphatics in aging and Alzheimer’s disease
Recent research from the Kipnis laboratory (1-3) has highlighted the significance of meningeal lymphatics, which were originally identified in the dura mater of rat (4). These investigators underscored the crucial role of the meningeal lymphatics in brain immune surveillance and the clearance of metabolic wastes. They have also mapped out the path and the types of macromolecules that efflux through the meningeal lymphatics when cerebrospinal fluid (CSF) drains out towards the deep cervical lymph nodes (dCLNs). In addition, they have modeled Alzheimer’s disease (AD) in mice utilizing amyloid β (Aβ) clearance as a measure of functionality of the meningeal lymphatics.
The distinction between age-related changes and the effects of a disease are not always well-defined (5). It is well known that aging affects neurodegenerative disorders such as AD (5) and human immunodeficiency virus 1 (HIV) infection of the central nervous system (CNS) (6). A critical role in the age associated changes may be ascribed to the progressive deterioration of the meningeal lymphatics. This decline in the functionality of meningeal lymphatics impacts the removal of excess fluid, metabolic wastes, small molecules, abnormally folded proteins and immune cells from the CNS into the dCLNs (2). In AD, Aβ proteins aggregate into amyloid fibrils. These fibrils accumulate as intracellular inclusions and are deposited into plaques. This hallmark feature of AD (7) may be further aggravated due to age-related deterioration of the meningeal lymphatics. Furthermore, Aβ plaques are increased in the brains of HIV-infected individuals. The HIV trans-activator of transcription (TAT) affects formation of Aβ protein via several indirect mechanisms. In presence of TAT, uniform amyloid fibrils convert into double-twisted fibrils and form populations of thick unstructured filaments and aggregates. HIV Tat and Aβ aggregates and complexes synergistically induce neurotoxicity (8). HIV and simian immunodeficiency virus reservoirs in the cervical lymph nodes also highlights the crucial role of the meningeal lymphatic system (9-11). These observations implicate the overlapping, yet distinct nature of two neurodegenerative diseases and the central role of aging in meningeal lymphatics.
In the article, “Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease” Mesquita and coworkers evaluated age associated impairments of meningeal lymphatics (1). Impairment was predicted to result in accumulation of Aβ, thereby diminishing cognitive ability, decreasing the efflux of interstitial fluid macromolecules, and decreasing the influx of CSF macromolecules. The authors utitlized several methods of examining and augmenting the contribution of meningeal lymphatics to Aβ clearance and amelioration of AD symptoms (1).
Data reported by Mesquita and coworkers demonstrated that the impairment of meningeal lymphatics worsens brain perfusion and leads to diminished cognitive ability along with an increase in Aβ accumulation. These observations further suggest that the buildup of macromolecules may be one of the mechanisms through which meningeal lymphatics contributes to AD. Interestingly, AD affected patients display Aβ pathology similar to the pharmacologically ablated mice. These results highlight the utility of such commonality to model AD in mice. It might be possible to test various therapeutics for the amyloid-beta pathology of AD, since the authors utilized vascular endothelial growth factor C (VEGF-C) to prevent or even reverse the amyloid pathology.
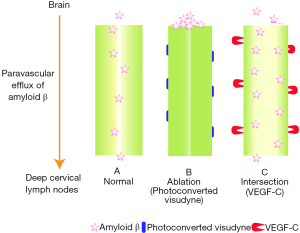
Meningeal lymphatics were impaired by (I) pharmacological ablation through the injection and photo conversion of Visudyne (also known as verteporfin); (II) surgical ligation of vessels that were afferent to the dCLNs; and (III) the development of Prospero homeobox protein 1 heterozygous mice that modeled meningeal lymphatic dysfunction (Figure 1). These impairment strategies resulted in a significant reduction of CSF drainage with no off-target effect as determined by the CSF tracer OVA-A647 (1). While all three impairment strategies were effective, only pharmacologically ablated mice were utilized for the vast majority of the experiments. Using this pharmacological ablation strategy, the authors performed magnetic resonance imaging (MRI) to assess meningeal lymphatic dysfunction and CSF perfusion. A reduction in influx into the hippocampus and cortex was inferred based exclusively on this single impairment strategy. Furthermore, RNA analysis, comparison with human subjects, and nearly all behavioral tests utilized in this study were also performed only with the pharmacologically ablated mice.
There are several additional ablation methods available to study meningeal lymphatics that were not utilized in this study (3). For example, pharmacological and genetic methods have been mixed by administering diphtheria toxin to Cre-lox mice that selectively express the human diphtheria toxin receptor on lymphatic endothelial cells through a lymphatic specific promoter (FLT-4) (12). Careful considerations in selecting ablation method is crucial when studying age-related neurological disorders as certain methods may result in relatively sudden large-scale disruption which may not recapitulate the effects of aging. When experimental disruption is not gradual, it is unlikely that the model will reflect what would happen in nature. Thus, any observations attributed to aging might be questionable.
Mesquita and coworkers utilized several AD mouse models such as 5XFAD and J20 transgenic mice for the pharmacological ablation studies. Hence, they claimed to provide a new AD mouse model. Of note, the reduction in CSF outflow aggravated Aβ accumulation as observed in AD patients. However, Aβ pathology is not the only hallmark of AD. For example, neurofibrillary tangles and loss of synapses correlate with dementia and cognitive decline, respectively (13). Whether dysfunction of meningeal lymphatics directly affects dementia or cognitive declines remains to be investigated.
In order to assess meningeal lymphatics dysfunction, Mesquita and coworkers (1) utilized the following behavior tests: (I) open field test (OFT); (II) novel location recognition (NLR); (III) contextual fear conditioning (CFC); and (IV) Morris water maze (MWM). Significant differences were only found in CFC, and the spatial learning portion of MWM. Notably, mice have an aversion to water and both tests thus have an inherent connection to fear. Authors did not take this into consideration while inferring their results. Additionally, outcomes of the behavior studies are not as expected in AD models. For example, in the OFT, locomotion is expected to increase as Alzheimer’s pathology intensifies. On the other hand, habituation is expected to decrease as compared to wild type controls (14). When the NLR test was utilized, lower recognition levels were noted in the 5XFAD transgenic mice with five familial AD mutations (15). It is unlikely that all forms of learning and memory were affected by ablation even though Aβ accumulation was affected. Several common behavioral tests that are often used for AD models, such as the Radial Arm Maze, the Radial Arm Water Maze, Passive-Avoidance Learning, the Y Maze, the T Maze, and Novel Object Recognition tests were not utilized (16). Therefore, further evaluation is necessary to determine additional factors besides Aβ accumulation that may contribute to memory deficits that are characteristic of AD and other forms of dementia.
VEGF-C may have therapeutic value in treating age-related neurodegenerative diseases in which meningeal lymphatic dysfunction has a critical role. VEGF-C can increase meningeal lymphatic vessel diameter while also improving peripheral lymphatic sprouting and function (17,18). Hence, the authors sought to compare the effect of this treatment in both young (2–3 months of age) and old (20–24 months of age) mice, utilizing an adeno-associated virus serotype 1 vector to induce expression of VEGF-C. Enhanced green fluorescent protein was utilized as a positive control. VEGF-C resulted in a significant increase in meningeal lymphatic vessel diameter without affecting blood vessel coverage in both young and old mice. Old mice also exhibited improved drainage into the dCLNs. Interestingly, only old mice experienced significant improvement in spatial learning and memory as determined with the MWM and NLR tests. These results suggest that there is a limit to the degree by which lymphatics can intensify functioning. However, the exclusion of other behavioral tests left an intriguing gap.
VEGF-C can increase neural stem cells in the hippocampus (19). However, no significant changes in the number of Ki-67+ cells were observed in the hippocampal dentate gyrus between mice with eGFP and VEGF-C viral vectors. Hence, the observed behavioral improvements were dependent upon improved lymphatics and not hippocampal neurogenesis. Meningeal lymphatics were further implicated to be the cause of these behavioral improvements when either VEGF-C or eGFP was administered while also ligating the lymphatic vessels that were afferent to dCLNs. Learning and memory were once again evaluated through behavioral tests in these mice. This time however, the beneficial effects that VEGF-C appeared to induce in the MWM and NLR tests seemed to completely disappear, thus supporting the authors’ hypothesis.
Taken together, this study suggests that meningeal lymphatic dysfunction plays a critical role in age-related changes in AD and that VEGF-C can alleviate this impairment to reduce cognitive inhibition. This may also be applicable to traumatic brain injuries, which are known to initiate amyloid pathologies that may be linked to developing AD later in life (20). As such, meningeal lymphatics represents a new method of combating amyloid pathology. Future research should also consider taking advantage of meningeal lymphatics as a novel method of drug delivery, particularly for enzymes that could break down amyloid plaque. This system may also be utilized to aid in prognosis, because amyloid seems to accumulate when the meningeal lymphatic system ceases to function, allowing tracers such as OVA-A647 to be administered to identify vulnerable areas of this pathology. Given how effective the pharmacological ablation method has proven to be in developing improved mouse models, future research should consider applying it to mouse models of other amyloid-related illnesses, such as Huntington’s disease or traumatic brain injury.
Acknowledgements
We would like to thank Robin Taylor for assistance with the illustration.
Funding: This work is partially supported by R21MH11345501 to SN Byrareddy.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018;560:185-91. [Crossref] [PubMed]
- Da Mesquita S, Fu Z, Kipnis J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 2018;100:375-88. [Crossref] [PubMed]
- Louveau A, Herz J, Alme MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 2018;21:1380-91. [Crossref] [PubMed]
- Andres KH, von During M, Muszynski K, et al. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 1987;175:289-301. [Crossref] [PubMed]
- Fjell AM, McEvoy L, Holland D, et al. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol 2014;117:20-40. [Crossref] [PubMed]
- Barrett L, Fowke KR, Grant MD. Cytomegalovirus, aging, and HIV: a perfect storm. AIDS Rev 2012;14:159-67. [PubMed]
- Iadanza MG, Jackson MP, Hewitt EW, et al. A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol 2018;19:755-73. [Crossref] [PubMed]
- Hategan A, Bianchet MA, Steiner J, et al. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 2017;24:379-86. [Crossref] [PubMed]
- Dave RS, Sharma RK, Muir RR, et al. FDC:TFH Interactions within Cervical Lymph Nodes of SIV-Infected Rhesus Macaques. J Neuroimmune Pharmacol 2018;13:204-18. [Crossref] [PubMed]
- Dave RS, Jain P, Byrareddy SN. Functional Meningeal Lymphatics and Cerebrospinal Fluid Outflow. J Neuroimmune Pharmacol 2018;13:123-5. [Crossref] [PubMed]
- Dave RS, Jain P, Byrareddy SN. Follicular Dendritic Cells of Lymph Nodes as Human Immunodeficiency Virus/Simian Immunodeficiency Virus Reservoirs and Insights on Cervical Lymph Node. Front Immunol 2018;9:805. [Crossref] [PubMed]
- Gardenier JC, Hespe GE, Kataru RP, et al. Diphtheria toxin-mediated ablation of lymphatic endothelial cells results in progressive lymphedema. JCI Insight 2016;1:e84095. [Crossref] [PubMed]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992;42:631-9. [Crossref] [PubMed]
- Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 2013;493:674-8. [Crossref] [PubMed]
- Park JC, Ma J, Jeon WK, et al. Fructus mume extracts alleviate cognitive impairments in 5XFAD transgenic mice. BMC Complement Altern Med 2016;16:54. [Crossref] [PubMed]
- Bryan KJ, Lee H, Perry G, et al. Transgenic Mouse Models of Alzheimer’s Disease: Behavioral Testing and Considerations. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience, 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis, 2009.
- Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A 2001;98:12677-82. [Crossref] [PubMed]
- Saaristo A, Veikkola T, Tammela T, et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med 2002;196:719-30. [Crossref] [PubMed]
- Han J, Calvo CF, Kang TH, et al. Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell Rep 2015;10:1158-72. [Crossref] [PubMed]
- Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci 2010;11:361-70. [Crossref] [PubMed]


