Medium-term outcome of cementless, mobile-bearing, unicompartmental knee arthroplasty
Introduction
Unicompartmental arthroplasty (UKA) is a viable alternative to total knee arthroplasty (TKA) in select patients (1-3). Although it is a successful procedure for the treatment of focal osteoarthritis or osteonecrosis of the medial or lateral compartments of the knee (4-6), the use of UKA in the management of unicompartmental arthritis remains controversial (7). An increased incidence of failure of UKA compared to TKA has been reported as a result of aseptic loosening and progression of arthritis in the unresurfaced compartments (8), and has led to the recommendation of TKA over UKA for the treatment of unicompartmental knee arthritis (2,9,10). With regard to conversion of UKA to TKA, the clinical outcomes and survival rates are significantly worse than primary TKA, and are more comparable to the results achieved with revision TKA (11-14). Nevertheless, better functional outcomes, along with a faster recovery, greater patient satisfaction, and lower risk of perioperative complications are associated with the use of UKA as compared to TKA (3,4,8,15-19).
One of the most commonly used, mobile-bearing UKA is the Oxford UKA (OUKA, Zimmer Biomet, Warsaw, IN, United States). Similar to other UKAs, the main reason for OUKA revision is aseptic loosening according to the registry data (20). Although radiolucent lines (RLLs) are frequently observed adjacent to the tibial component in well-functioning cemented OUKAs (21), some authors debate whether these “physiological” RLLs are indicative of loosening, and consequently result in unnecessary revision procedures (22).
A cemented version of OUKA has been in use for over 30 years, and in 2003 a cementless version was introduced, which has rapidly gained popularity. Putative advantages of cementless fixation are shorter surgical time, avoidance of cementation errors, and a lower incidence of RLLs (23). A multicentre study including the developing hospital has reported significantly fewer RLLs for the cementless OUKA compared with the cemented version, with equivalent functional outcomes at 1- and 5-year follow-up (22).
Our independent study reports the results of a cohort at 5 years, with an emphasis on clinical outcomes. We hypothesize that the device would yield favourable functional outcomes, with a low incidence of RLLs and a lower revision rate than that reported in the registries for the cemented OUKA.
Methods
We conducted a retrospective study of cementless OUKA performed in an orthopaedic centre specializing in knee arthroplasty and sports medicine in a consecutive series of patients. From April 2009 to February 2014, 186 mobile-bearing OUKA were performed in our centre, of which 153 procedures (150 patients) were cementless. Indications for OUKA included primary anteromedial osteoarthritis, intact cruciate ligaments, intra-articular correctable varus deformity, preservation of the lateral compartment, flexion deformity of less than 15°, and ability to flex at least 110° under anaesthetic (2).
The mean age of the study population at operation was 70.6±7.5 years (range, 54–86 years). Of these patients, 110 (73.3%) were female and 40 were male (26.7%). The mean body mass index was 30.4 kg/m2 (range, 24.8–34.6 kg/m2). The most frequent diagnosis was primary osteoarthritis in 142 knees (140 patients), followed by posttraumatic arthrosis in 8 knees (8 patients), and rheumatoid arthritis in 3 knees (2 patients). One patient had undergone reconstruction of the anterior cruciate ligament six months prior to OUKA. None of the patients had a prior high tibial osteotomy.
All procedures were performed by a single surgeon (RS). After completion of a short medial arthrotomy, a thorough inspection of the knee was completed to make a final decision about patient eligibility for cementless OUKA based on an assessment of the ligamentous stability, an intraoperative visual inspection of bone quality, and on a Bone Hardness Test (BHT). Exercising pressure with the thumb (or the index finger in case of a small knee) on the surface created after resection of the tibia allowed the surgeon to assess the hardness of bone tissue. If the pressure exerted on the bone caused the deflection of resected surface (i.e., if the thumb delved into the bone tissue), the hardness of bone was not deemed to be sufficient to provide primary stability of the implant, and a cementless implant was not used. Based on the intraoperative subjective appraisal of bone quality, 29 patients received a cemented OUKA. These patients were excluded from the study. Care was taken to resect as minimal tibial bone as possible, as the strength of the cancellous bone decreases with increasing depth (24,25). Phase 3 instrumentation was used in the beginning of the study. Later, microplasty instrumentation was utilized. The mean operative time (skin-to-skin) in the study group was 57 minutes (range, 45–100 minutes). A tourniquet was used in all cases.
Postoperative pain management consisted of morphine, tramadol, and paracetamol (IV) and diclofenac sodium, pyralginum, and ketoprofen (IM). All patients received thrombosis prophylaxis with fraxiparine for 2 to 4 weeks, depending on the co-morbidity status of the patient.
Patients were instructed to begin active knee flexion and extension as soon as possible after surgery. Full weight-bearing exercise with the aid of two elbow crutches was started on the first postoperative day. Full extension or 90 degrees flexion was the goal at discharge, and patients who could not achieve this began immediate physical therapy. All other patients started physical therapy 8 weeks postoperatively, and were monitored to ensure adequate wound healing within the first 2 weeks.
Function, range of motion, and pain were assessed by the Knee Society Score (KSS) (26) and the Oxford Knee Score (OKS) (27), which were administered at baseline and at final follow-up. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores (28) and Forgotten Joint Scores (29) were obtained at the final follow-up.
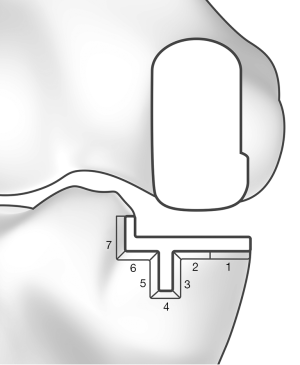
Because of the observational character of the study, patients were not examined radiographically at final follow-up. However, a substantial number of patients (n=77, 78 knees) brought their own radiographs, which were taken by their general practitioner. All available radiographs were examined for the presence of RLLs and osteolysis, implant loosening, and migration. The area under the tibial tray was divided into 7 zones (Figure 1) (13). Radiolucent lines were classified as physiological if they were less than 2 mm in depth, had a sclerotic demarcation, and if they appeared within the first 12 postoperative months. The radiolucent lines were deemed pathological if they were thick, progressive, and without sclerotic demarcation (30). Intra- and preoperative complications up to discharge were recorded, alongside postoperative complications up to the latest follow-up.
Informed consent was obtained from all individual participants included in the study. In accordance with the legislation of the country where the study was conducted, ethics committee approval was not obtained, as the study was purely observational, with no changes to standard clinical practice.
Continuous data are presented as the mean and range. Categorical variables are presented as frequencies and percentages. Implant survivorship was calculated using Kaplan-Meier analysis (31), with the revision of any component for any reason as event of interest. Exchange of the polyethylene (PE) meniscal was classified under reoperation. Kaplan-Meier analysis was performed with patients for whom the revision status was known. Pearson’s chi-square test and student t-test were used for analysis. Stata 12.1 (StataCorp, College Station, TX, USA) was used for analysis.
Results
At final follow-up, 150 knees (147 patients) could be assessed clinically, at a mean follow-up of 5±1.3 years (range, 3–7 years) (Table 1). No patients were lost to follow-up. Radiographic follow-up was performed in 78 knees (77 patients). Three patients (3 knees) died during the course of the study for unrelated reasons at 12, 14, and 24 months. All patients had an excellent clinical and radiographic outcome at their last follow-up.
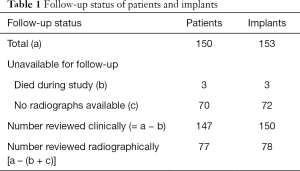
Full table
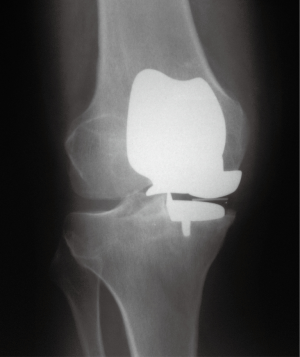
Overall, none of the prostheses were explanted. Two knees (2 patients) required exchange of the bearing surface after 4 months and 4 years postoperatively due to dislocation of the polyethylene insert. In one patient, the dislocation had occurred while standing up from a sitting position. The second patient experienced a dislocation while picking up a heavy object from the floor. After exchanging the insert with a thicker polyethylene insert, the patient recovered without sequelae. In another patient, patellofemoral arthroplasty was performed 2 years postoperatively due to painful progression of the osteoarthritis to the patellofemoral compartment (Figure 2). No intraoperative or postoperative complications were noted in any other of the patients.
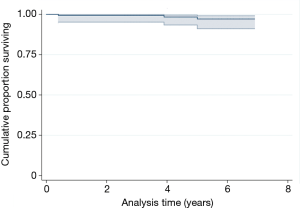
Survival for aseptic loosening of the device was 100% after 5 years. With reoperation for any reason as endpoint of interest, the survival rate was 97.1% (95% CI, 91.1–99.1%) (Figure 3). Clinical status improved markedly between the preoperative visit and the latest follow-up. The mean KSS knee and function score improved from 35.2±13.6 (range, 8–38) and 33.2±10.6 (range, 5–50) preoperatively to 85.9±8.6 (range, 84.5–87.3) and 76.3±12.4 (range, 15–100) at final follow-up. The mean OKS improved from 15.1±2.9 (range, 11–22) to 38.3±3.9 (range, 24–46). WOMAC scores improved from 34.8±4.1 (range, 17–41) to 76.6±11.6 (range, 42–88). At final follow-up, the FJS-12 was 75.5±5.5 (range, 65–96).
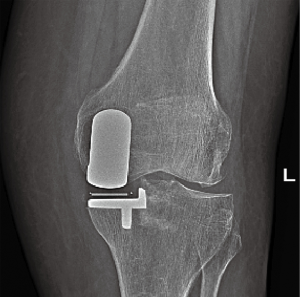
Radiographic assessment yielded favourable findings in most of the cases (Figure 4). In 78 knees (77 patients) with postoperative radiographic data, 1 knee showed progression of osteoarthritis in the lateral compartment in a clinically asymptomatic patient. RLLs were observed in 8 knees (10.3%), of which 5 (6.4%) were located in tibial zone 7 adjacent to the vertical wall of the tibial baseplate. In addition, 1 knee (1.3%) showed a partial RLL in tibial zones 1, 2, and 6, and in 1 knee RLLs were noted in tibial zones 1 and 2. One patient showed a RLL in the posterior aspect of the femoral component. There were no complete RLLs. All RLLs were small (<1 mm), and none of the RLLs was classified as pathological.
Conclusions
The cementless OUKA was developed in response to the reported rate of RLLs, with the goal of reducing revision rates and increasing longevity of the implant. Only a few reports describing medium-term outcome after cementless OUKA from independent centres have been published. In our study, no aseptic loosening was found and overall reoperation rates remain low with favourable functional scores and radiological findings 5 years after surgery. Our results are similar to those from the designers’ centre (22,30,32) and to the results from other independent centres (13,33), and they are slightly better than those presented by another independent centre (34). We attribute this to the adherence to the recommended indications and techniques.
Patient-reported scores indicate better outcome in UKA than in TKA (13,35) despite higher revision rates consistently reported in registry data (2,20,36). Additionally, patients who have undergone medial UKA have reported less awareness of their artificial joint than patients who have undergone TKA (37). Our results show good clinical outcomes on both the OKS and FJS-12. In this study, OKS compared favourably with the 6-month results for TKA from the National Joint Registry for England and Wales (38) and the New Zealand Joint Registry (11). FJS-12 in our OUKA cohort was significantly higher than that reported in a cohort of TKA patients by Behrend et al. (29) and by Zuiderbaan et al. (37). Our study results were comparable to the cohort of UKA by Zuiderbaan et al. (37) and nearly equivalent to results reported in a healthy group of controls reported by Behrend et al. (29) (Table 2). We hypothesize that cementless UKA leads to a “forgotten joint”; however, further research is warranted to investigate this.

Full table
The absence of aseptic loosening in our study population support the findings of Liddle et al. who showed a relationship between revision rate and percentage of knee arthroplasties that are UKA (39). With increasing use of UKA to around 50%, there is a steady decrease in the revision rate (39). When UKA is selected in 40% to 60% of patients, the revision rate was found not to be significantly different from TKA. Within the timeframe of the study, the senior author of this paper (RS) has implanted UKA in nearly 50% of his patients.
In our study, no revisions of the tibial or femoral component were required. RLLs were present in 8 out of 78 cases; 5 cases showed RLLs adjacent to the lateral wall only. There were no complete RLLs, and only a single case with a partial femoral RLL. In the 2 cases with horizontal tibial RLLs, one was small and located in the peripheral zones 1 and 2, whereas the other one extended into zone 6. Radiography did not show any signs of loosening, and clinical outcome was favourable in both cases. In all cases, all components appeared securely fixed. The incidence of RLL observed in the present study is significantly lower than the incidence reported for cemented OUKA (up to 70%) (21,40). Our results are similar to the findings of other studies of the cementless implant (13,22,30).
The design of the OUKA prosthesis allows ingrowth of bone onto the porous titanium and calcium hydroxyapatite (HA) coating. Specifically, the vertical wall on the lateral aspect of the tibial implant is HA coated, but it is not coated with porous titanium and it is not specifically designed for bony ingrowth since this area is considered non-weightbearing. Despite this, all patients who were radiographically assessed showed at least partial ingrowth in all knees. As such, we agree with Hooper et al., who suggested a porous titanium coating may not be necessary for bony ingrowth in this non-weightbearing zone (13).
There are several limitations to our study, which includes its retrospective, observational study design. For a large proportion of patients, no radiographs were available, and radiographs were not taken in a standardized manner. In addition, our results were not compared with those for other UKA systems or with those achieved with TKA.
Other study limitations are the lack of long-leg, weight-bearing radiographic views to assess overall postoperative alignment and an absence of follow-up radiographs taken under fluoroscopic control (41). Despite the missing radiographs, alignment of all implants was assessed clinically at the time of the follow-up with no reported instance of ongoing varus or valgus malalignment. It is important to note that during surgery there was no overcorrection and no attempt made to position the knee in physiological valgus.
Three patients (3 knees) died during the course of the study. The rate of attrition from the initial study sample can be considered as being low (2%), which adds to the credibility of our findings.
Our clinical observations of this elderly cohort show that OUKA performs well in those with osteoporotic bone. Prior studies have reported peri-prosthetic fracture not encountered with the cemented component (22), and while this incidence was not significant there could still be increased concern in implanting this prosthesis in the elderly population. However, our current study results support our policy of offering patients a cementless medial OUKA if they fulfil the operative criteria without excluding patients because of age, gender, or bone density. The only criterion that we used intraoperatively was visual perception and the Bone Hardness Test. Based on this simple algorithm, only 15.9% (29/182) of the knees were deemed ineligible for cementless OUKA. In conclusion, we have shown excellent functional outcomes from early results at 5 years with the cementless OUKA. In this patient population, we demonstrated a very low incidence of RLLs and overall component survivorship of 100% at 5 years.
Acknowledgements
None.
Footnote
Conflicts of Interest: Funding for manuscript development was provided by Zimmer GmbH, Winterthur, Switzerland. The authors received no payments. The funding party had no involvement in the writing of the report or in the decision to submit the results for publication. On behalf of all authors, the corresponding author states that there is no conflict of interest. Dr. R Stempin is consultant for Zimmer Biomet. Dr. K Stempin and Dr. W Kaczmarek have no conflicts of interest to declare.
References
- Redish MH, Fennema P. Good results with minimally invasive unicompartmental knee resurfacing after 10-year follow-up. Eur J Orthop Surg Traumatol 2018;28:959-65. [Crossref] [PubMed]
- Murray DW, Liddle A, Dodd CA, et al. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J 2015;97-b:3-8.
- Liddle AD, Judge A, Pandit H, et al. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet 2014;348:1437-45. [Crossref] [PubMed]
- Newman J, Pydisetty R, Ackroyd C. Unicompartmental or total knee replacement: the 15-year results of a prospective randomised controlled trial. J Bone Joint Surg Br 2009;91:52-7. [Crossref] [PubMed]
- Price AJ, Svard U. A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res 2011;469:174-9. [Crossref] [PubMed]
- Xue H, Tu Y, Ma T, et al. Up to twelve year follow-up of the Oxford phase three unicompartmental knee replacement in China: seven hundred and eight knees from an independent centre. Int Orthop 2017;41:1571-7. [Crossref] [PubMed]
- Chatellard R, Sauleau V, Colmar M, et al. Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res 2013;99:S219-25. [Crossref] [PubMed]
- Arirachakaran A, Choowit P, Putananon C, et al. Is unicompartmental knee arthroplasty (UKA) superior to total knee arthroplasty (TKA)? A systematic review and meta-analysis of randomized controlled trial. Eur J Orthop Surg Traumatol 2015;25:799-806. [Crossref] [PubMed]
- Lyons MC, MacDonald SJ, Somerville LE, et al. Unicompartmental versus total knee arthroplasty database analysis: is there a winner? Clin Orthop Relat Res 2012;470:84-90. [Crossref] [PubMed]
- Koskinen E, Eskelinen A, Paavolainen P, et al. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop 2008;79:499-507. [Crossref] [PubMed]
- Pearse AJ, Hooper GJ, Rothwell A, et al. Survival and functional outcome after revision of a unicompartmental to a total knee replacement: the New Zealand National Joint Registry. J Bone Joint Surg Br 2010;92:508-12. [Crossref] [PubMed]
- Wynn Jones H, Chan W, Harrison T, et al. Revision of medial Oxford unicompartmental knee replacement to a total knee replacement: similar to a primary? Knee 2012;19:339-43. [Crossref] [PubMed]
- Hooper N, Snell D, Hooper G, et al. The five-year radiological results of the uncemented Oxford medial compartment knee arthroplasty. Bone Joint J 2015;97-B:1358-63. [Crossref] [PubMed]
- Thienpont E. Conversion of a unicompartmental knee arthroplasty to a total knee arthroplasty: can we achieve a primary result? Bone Joint J 2017;99-b:65-9.
- Murray DW, Goodfellow JW, O'Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg Br 1998;80:983-9. [Crossref] [PubMed]
- Felts E, Parratte S, Pauly V, et al. Function and quality of life following medial unicompartmental knee arthroplasty in patients 60 years of age or younger. Orthop Traumatol Surg Res 2010;96:861-7. [Crossref] [PubMed]
- Noticewala MS, Geller JA, Lee JH, et al. Unicompartmental knee arthroplasty relieves pain and improves function more than total knee arthroplasty. J Arthroplasty 2012;27:99-105. [Crossref] [PubMed]
- Wiik AV, Aqil A, Tankard S, et al. Downhill walking gait pattern discriminates between types of knee arthroplasty: improved physiological knee functionality in UKA versus TKA. Knee Surg Sports Traumatol Arthrosc 2015;23:1748-55. [Crossref] [PubMed]
- Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty 2012;27:86-90. [Crossref] [PubMed]
- National Joint Registry for England W, Northern Ireland and the Isle of Man. 13th Annual Report. Adelaide: National Joint Registry; 2016.
- Pandit H, Jenkins C, Barker K, et al. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br 2006;88:54-60. [Crossref] [PubMed]
- Liddle AD, Pandit H, O'Brien S, et al. Cementless fixation in Oxford unicompartmental knee replacement: a multicentre study of 1000 knees. Bone Joint J 2013;95-B:181-7. [Crossref] [PubMed]
- Campi S, Pandit HG, Dodd CA, et al. Cementless fixation in medial unicompartmental knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2017;25:736-45. [Crossref] [PubMed]
- Hvid I. Trabecular bone strength at the knee. Clin Orthop Relat Res 1988.210-21. [PubMed]
- Scott CEH, Eaton MJ, Nutton RW, et al. Metal-backed versus all-polyethylene unicompartmental knee arthroplasty: Proximal tibial strain in an experimentally validated finite element model. Bone Joint Res 2017;6:22-30. [Crossref] [PubMed]
- Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res 1989.9-12. [PubMed]
- Dawson J, Fitzpatrick R, Murray D, et al. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63-9. [Crossref] [PubMed]
- Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40. [PubMed]
- Behrend H, Giesinger K, Giesinger JM, et al. The "forgotten joint" as the ultimate goal in joint arthroplasty: validation of a new patient-reported outcome measure. J Arthroplasty 2012;27:430-6.e1. [Crossref] [PubMed]
- Pandit H, Liddle AD, Kendrick BJ, et al. Improved fixation in cementless unicompartmental knee replacement: five-year results of a randomized controlled trial. J Bone Joint Surg Am 2013;95:1365-72. [Crossref] [PubMed]
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Campi S, Pandit H, Hooper G, et al. Ten-year survival and seven-year functional results of cementless Oxford unicompartmental knee replacement: A prospective consecutive series of our first 1000 cases. Knee 2018;25:1231-7. [Crossref] [PubMed]
- Campi S, Pandit HG, Oosthuizen CR. The Oxford Medial Unicompartmental Knee Arthroplasty: The South African Experience. J Arthroplasty 2018;33:1727-31. [Crossref] [PubMed]
- Panzram B, Bertlich I, Reiner T, et al. Cementless Oxford medial unicompartimental knee replacement: an independent series with a 5-year-follow-up. Arch Orthop Trauma Surg 2017;137:1011-7. [Crossref] [PubMed]
- Rothwell AG, Hooper GJ, Hobbs A, et al. An analysis of the Oxford hip and knee scores and their relationship to early joint revision in the New Zealand Joint Registry. J Bone Joint Surg Br 2010;92:413-8. [Crossref] [PubMed]
- Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. Adelaide: AOA; 2016.
- Zuiderbaan HA, van der List JP, Khamaisy S, et al. Unicompartmental knee arthroplasty versus total knee arthroplasty: Which type of artificial joint do patients forget? Knee Surg Sports Traumatol Arthrosc 2017;25:681-6. [Crossref] [PubMed]
- Liddle AD, Pandit H, Judge A, et al. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14 076 matched patients from the National Joint Registry for England and Wales. Bone Joint J 2015;97-B:793-801. [Crossref] [PubMed]
- Liddle AD, Pandit H, Judge A, et al. Optimal usage of unicompartmental knee arthroplasty: a study of 41 986 cases from the National Joint Registry for England and Wales. Bone Joint J 2015;97-B:1506-11. [Crossref] [PubMed]
- Gulati A, Chau R, Pandit HG, et al. The incidence of physiological radiolucency following Oxford unicompartmental knee replacement and its relationship to outcome. J Bone Joint Surg Br 2009;91:896-902. [Crossref] [PubMed]
- Vyskocil P, Gerber C, Bamert P. Radiolucent lines and component stability in knee arthroplasty. Standard versus fluoroscopically-assisted radiographs. J Bone Joint Surg Br 1999;81:24-6. [Crossref] [PubMed]