Efficacy and safety of the Fu-Zheng-Qu-Zhuo method on retarding the progress of chronic kidney disease (stage 3–4): a systematic review and meta-analysis
Introduction
Chronic kidney disease (CKD) is a substantial worldwide clinical and public health problem, linked to high health care costs, poor quality of life and serious adverse health outcomes (1-4). In China, the prevalence of CKD reported by Zhang et al. was 10.8% and stage 3–4 CKD was 1.7% (5). Thus, it is necessary to discover more effective therapies to retard the progress of CKD, avoiding the occurrence of end stage renal disease (ESRD).
The treatment of CKD by a traditional Chinese medicine (TCM) method has a long history in China (6). An increasing amount of studies has investigated the efficacy of TCM on CKD; however, there still remains controversy concerning this treatment. To our knowledge, there is no review which has chosen composite endpoint events (CEP) as the primary outcome to evaluate the efficacy of TCM on stage 3–4 CKD. Therefore, we have conducted this review to evaluate the efficacy and safety of TCM on retarding the progress of stage 3–4 CKD.
Methods
This meta-analysis was conducted according to the recommendations and checklist from the preferred reporting items for systematic review and meta-analysis (PRISMA) statement (7).
Search strategy
We searched the relevant randomized controlled trials (RCTs) from the Medline, Cochrane Library, Embase, SinoMed, Wanfang, CNKI, and Weipu (VIP) databases from the inception to June 2018. Conference proceedings, and reference lists of relevant articles were also searched.
Eligibility criteria of original studies
Inclusion criteria: (I) participants: we included adult patients with CKD (stage 3–4) who did not receive dialysis, and with the diagnostic criteria that was explicit and normative, regardless of the cause, gender and ethnicity; (II) interventions: Chinese herbal compound combined with integrated therapy; (III) control: integrated therapy; (IV) outcomes: (i) primary outcome: CEP; (ii) secondary outcomes: progress of CKD, cardiovascular events, the incidence of hyperkalemia, 24-h urine protein (24-h UP), albumin (ALB), hemoglobin (HB); (V) the study design was a RCT.
Exclusion criteria: (I) a study that administered Niaoduqing particles as a control; (II) a study with duplicate publication and/or abstract only.
Study selection
Two reviewers independently identified studies through inclusion criteria by screening the title and abstract of each record and retrieved their full-text if necessary. Any disagreement between the two reviewers was solved with a discussion with a third reviewer. Otherwise, the agreement was accomplished by a consensus.
Data extraction and quality assessment
We designed a pre-defined data extraction form and two reviewers independently extracted the following information from the selected trials: the first author, published year, sample size, mean age, intervention, control, CKD stage, and outcomes. Any disagreement between the two reviewers was discussed with a third reviewer until a consensus was reached.
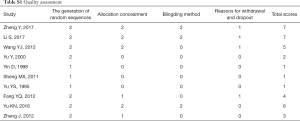
The quality of the RCT studies was assessed using a modified Jadad scale (8), including the generation of random sequences, allocation concealment, blinding method, and reasons for withdrawal and dropout at the time of follow-up. According to this system, a score of 1 to 3 indicated a low-quality study and a score of 4 to 7 indicated a high-quality study. The maximum for the Jadad score was 7.
Data synthesis
Stata software (version 11.0, Stata Corp LP, College Station, TX, USA) was used for statistical analysis. According to the Cochrane Handbook of Systematic Reviews, we chose risk ratios (RRs) and 95% confidence intervals (CIs) as the appropriate parameters to evaluate the dichotomous outcomes, such as CEP and the incidence of hyperkalemia. In terms of continuous outcomes, the mean difference and its 95% CI were used.
Between-study heterogeneity was evaluated using an I2 test (25% or lower is defined as low heterogeneity, 50% as moderate heterogeneity, 75% as high heterogeneity). The fixed-effect model was applied if there was no or low heterogeneity, and pooled RRs were estimated using the Mantel-Haenszel method. Publication bias was assessed if there are more than ten studies in one outcome. All hypotheses were tested at the alpha =0.05 level.
Results
Description of included studies
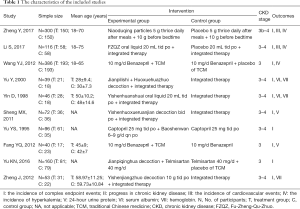
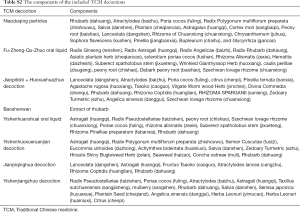
We identified 690 records based on this search strategy, and 338 potentially eligible records were obtained after removing duplicate publications. After screening the titles and abstracts, a total of 318 studies were excluded. Ten studies (9-18) with 1,308 participants were included and eight studies (9-14,16,18) included meta-analyses (Figure 1). The characteristics of the included studies are presented in Table 1. Risk of bias for the included studies was assessed by a modified Jadad scale, and the results are presented in Table S1. Four studies (9-11,16) reported the composite endpoint, and three (9-11) of the four were published in English. Wang et al. (11) and Fang et al. (16) included the participants with stage 3 CKD, while two other studies (9,10) included stage 3–4 CKD. The quantitative value of progress of CKD (b) in Sheng et al. (14) and Yu et al. (15) were positive in the treatment group and negative in the control group, while it was both negative in the two groups in the studies by Yu et al. (12) and Yin et al. (13). The components of the included TCM decoctions are shown in Table S2.
Full table
Full table
Full table
Quality assessment
Five studies (9-11,16,17) were high-quality studies with a Jadad score of 4 or higher, while the others were low-quality studies (Table S1). We found that most of the low-quality studies did poorly in allocation concealment and reasons for withdrawal and dropout at the time of follow-up. At the same time, it is interesting that four (9-11,16) of the five high-quality studies reported CEP, which indicated that it is important to choose endpoints for RCTs.
Primary outcome
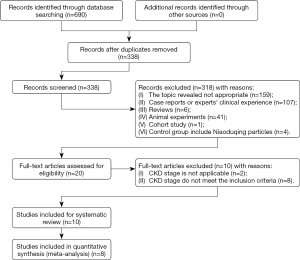
CEP was defined as the initiation of dialysis, CKD-related death, or the doubling of serum creatinine (Scr). Four studies (9-11,16) with 842 participants (418 participants were included in the treatment group and 424 participants in the control group) reported CEP. Compared with the control group, the occurrence of CEP was significantly reduced in the treatment group (RR =0.56, 95% CI: 0.33–0.94, P=0.029, I2=0.0%). There was no between-study heterogeneity (I2=0.0%) and the sensitivity analysis was not performed (Figure 2).
Secondary outcomes
There were four studies (12-15) with slope of the regression line (b) indicating the progress of CKD. The quantitative value of b in two studies (12,13) were negative. For the first study (12) (b=−0.41±0.28 in the treatment group and b=−1.24±0.72 in the control group), a significant statistical difference was detected with P<0.01. Meanwhile, the second study (13) (b=−0.41±0.28 in treatment group and b=−1.24±0.72 in control group), a significant statistical difference was detected with P<0.05. In the study of Sheng et al. (14), b=0.46±0.02 in the treatment group and b=−0.69±0.018 in the control group, for Yu et al. (15), b=−9.46 in the treatment group and b=−42.67 in the control group (Table S3).
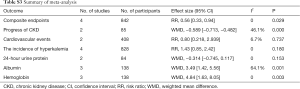
Full table
There were two studies (9,10) with 408 participants which had a record of cardiovascular events. One study (9) reported that there were three participants who had cardiovascular events that occurred in the treatment group and two in the control group. Another study (10) reported that there was one participant in the treatment group and three in the control group (Table S3).
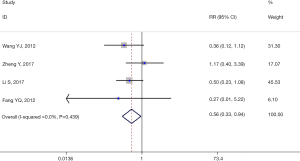
Four studies (9-11,16) containing 828 participants had a record of the incidence of hyperkalemia. No statistical difference was observed according to the meta-analysis between treatment groups and control groups (RR =1.43, 95% CI: 0.85–2.42, P=0.180, I2=0.0%). There was no between-study heterogeneity (I2=0%) and the sensitivity analysis was not performed (Figure 3).
There were two studies (14,16) which reported a 24-h urine protein; the first one recorded the data of 24-h UP (0.98±0.89 g in treatment group and 1.00±1.07 g in control group), while the second recorded the data of 24-h UP (1.348±1.404 g in treatment group and 1.670±0.720 g in control group). Both studies showed that there was no difference between the two groups (Table S3).
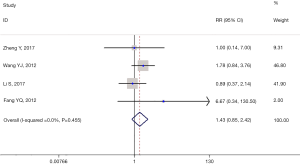
We performed analysis for three studies (12,13,18) which included 138 participants to investigate the change of ALB. Meta-analysis showed that significant differences were observed between the two groups (RR =3.49, 95% CI: 1.42–5.56, P=0.001, I2=64.1%) (Figure 4). However, between-study heterogeneity (I2=64%) indicated moderate heterogeneity. After sensitivity analysis, we found that when excluding the data from Zheng et al. (18), the heterogeneity was significantly decreased (I2 =0%). The result showed that (RR =4.49, 95% CI: 3.14–5.83, P<0.00001, I2=0%), which indicated that the main source of heterogeneity came from Zheng et al. When the full-text was screened, we found that calcium carbonate D3 and alfacalcidol were used both in the treatment group and the control group, which may have caused the heterogeneity. The total result of the meta-analysis was not influenced significantly before and after excluding the study by Zheng et al.
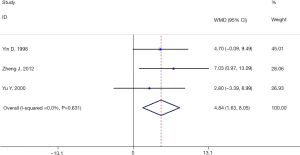
There were three studies (12,13,18) which reported the data of HB. Compared with the control group, the treatment group had significantly increased levels of HB (RR =4.84, 95% CI: 1.63–8.05, P=0.003, I2=0.0%) (Figure 5).
Strength of evidence
The GRADE approach was used to assess the quality of the evidence for each outcome. As shown in Table S4, the quality of evidence for most of the outcomes were moderate to low.
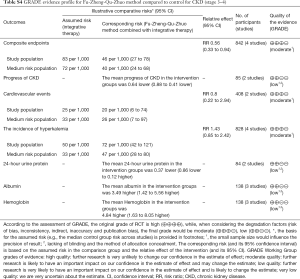
Full table
Discussion
Summary of the findings
To the best of our knowledge, this is the first systematic review choosing CEP and progress of CKD outcomes to assess TCM on retarding the progress of CKD (stage 3–4). It’s well known that stage 3–4 CKD is the key stage for patients, and, if treated inappropriately, a considerable proportion of the patients will progress to ESRD (resulting in dialysis treatment or death).
The main findings of this review demonstrate that by adding TCM based on integrated therapy, a decrease in the occurrence of CEP, and a retarding of the progress of stage 3–4 CKD can be achieved. In addition, this treatment method did not increase the risk of hyperkalemia, indicating that it is safe to use TCM for CKD, contrary to the majority of the findings from previous studies. Compared with integrated therapy alone, adding TCM could significantly increase the level of ALB and HB, suggesting that TCM may be retarding the progress of CKD by improving the function of the liver and spleen, which can further strengthen organic immunity. This treatment concept is similar to the opinion of Kalantar-Zadeh et al. (1). However, the treatment was not shown to decrease 24-h UP, which is also contrary to the majority of the findings from previous studies. It is interesting that, after screening the components carefully, we found that all of the TCM treatments included rhubarb (dahaung). In addition, most of the TCM treatments included tonics such as Astragalus (huangqi) and Angelica sinensis (danggui). Consequently, we named all of the TCM treatments we found “the Fu-Zheng-Qu-Zhuo method”.
Modern pharmacological studies show that Astragalus and Angelica sinensis have antifibrotic effects on obstructive nephropathy, and the molecular mechanism may relate to a decreased reactive oxygen species reaction (19). Recent studies have revealed that the colon is an important organ in the generation of uremic toxins. Colon-derived toxins not only promote CKD progression, but are also closely linked with mortality in patients with CKD. The above mechanism is similar to the theory of TCM. In TCM, the pathogenesis of CKD is a retention of toxins, and the common method to eliminate the toxins is through the colon, with rhubarb being the most common herb to use (20). Thus, rhubarb-based compounds could regulate intestine flora and reduce intestinally-derived uremic toxins produced by gut bacteria. In addition, Chinese researchers have found that, as the main active ingredient of rhubarb, emodin regulates lipopolysaccharide-induced toll-like receptor 4, and reduces the expression of tumor necrosis factor alpha and interleukin 6 (21).
Limitations of this review also exist. Since the slope of the regression line (b) had a negative and positive, we could not merge all of the data for analysis, which might have influenced the total results of the meta-analysis. In addition, the sample size and follow-up period were limited, which might also have impacted the results.
Expectation for further research
Clinical research, especially the domestic kind, tends to choose an effective rate as an outcome, which is equivocal evidence, and cannot solve practical clinical problems. Some researchers may choose the change of Scr level as the primary endpoint; however, due to Scr dependence on age, muscle mass, volume status, and renal haemodynamics, the changes in Scr related to treatment with diuretics or angiotensin-converting enzyme inhibitors are not necessarily associated with worse outcomes. In fact, the main cause of mortality for CKD are its serious adverse health outcomes. These include things like cardiovascular disease, infection, etc. Heart failure, for example, is common in CKD patients and is associated with a high morbidity and mortality rate (22). The interaction between cardiac and renal dysfunction may be critical for disease progression, since cardiac and renal dysfunction can cause mutual exacerbation through a variety of mechanisms such as fluid overload, hypo-perfusion and inflammatory activation (23-25). Thus, further studies should pay more attention to the outcomes of serious adverse health outcomes. In terms of clinical research by TCM, the stage of CKD should also be considered, since patients entering stage 5, have little opportunity for TCM use, whereas stage 3–4 patients might have more occasion to receive TCM treatment (10). Therefore, further TCM researchers should focus on stage 3–4 CKD, and choose the more serious adverse health outcomes as the primary outcome for evaluating the efficacy and safety of TCM on treating CKD.
Conclusions
In conclusion, the Fu-Zheng-Qu-Zhuo method combined with integrated therapy was found to decrease the occurrence of CEP, and retard the progress of stage 3–4 CKD. In addition, there was no increase in the risk of hyperkalemia. We recommend the use of the Fu-Zheng-Qu-Zhuo method combined with integrated therapy for stage 3–4 CKD.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (No. 81273747).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kalantar-Zadeh K, Fouque D. Nutritional Management of Chronic Kidney Disease. N Engl J Med 2018;378:584-5. [PubMed]
- Nugent RA, Fathima SF, Feigl AB, et al. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract 2011;118:c269-77. [Crossref] [PubMed]
- Bello AK, Nwankwo E, El Nahas AM. Prevention of chronic kidney disease: a global challenge. Kidney Int Suppl 2005.S11-7. [Crossref] [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [Crossref] [PubMed]
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815-22. [Crossref] [PubMed]
- Zhong Y, Deng Y, Chen Y, et al. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int 2013;84:1108-18. [Crossref] [PubMed]
- Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013;8:e83138. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Zheng Y, Cai GY, He LQ, et al. Efficacy and Safety of Niaoduqing Particles for Delaying Moderate-to-severe Renal Dysfunction: A Randomized, Double-blind, Placebo-controlled, Multicenter Clinical Study. Chin Med J (Engl) 2017;130:2402-9. [Crossref] [PubMed]
- Li S, Rao XR, Dai XW, et al. Beneficial effects of Fu-Zheng-Qu-Zhuo oral liquid combined with standard integrated therapy in patients with chronic kidney disease (stage 3-4): A randomized placebo-controlled clinical trial. Medicine (Baltimore) 2017;96:e7448. [Crossref] [PubMed]
- Wang YJ, He LQ, Sun W, et al. Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol 2012;139:757-64. [Crossref] [PubMed]
- Yu Y, Cui J, Zhang F. Chinese drugs for invigorating spleen to remove dampness, activating blood circulation to eliminate turbid in retarding progression of chronic renal failure. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000;20:727-8. [PubMed]
- Yin D, Dai X, Rao X. Yishen huanshuai recipe retard progression of chronic renal failure. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998;18:402-4. [PubMed]
- Sheng MX, Sun W, Xu LD, et al. Clinical study on delaying the pathological progress of chronic renal failure in the stage of nitrogenemia by invigorating the kidney and activating blood soft and firm. Journal of Shandong University of Traditional Chinese Medicine 2011:25-7.
- Yu YS, Li LS, Zhang X. Long-term effect of rhubarb on chronic renal failure. J Nephrol Dialy Transplant 1995;4:32-5.
- Fang YQ, Lu Y, Wang YJ. Efficiency of benazepril combined with wind dispelling and dampness removing chinese herbs on stage 3 chronic kidney disease with wind-dampness syndrome: a prospective study. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012;32:311-6. [PubMed]
- Yu KN, Ni ZH, Wang NS, et al. A Clinical Multicenter Randomized Controlled Study on JianpiQinghua Decoction in Treating Stage 3 Chronic Kidney Disease with A Syndrome Type of Dampness-heat due to Spleen Deficiency. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016;38:686-95. [PubMed]
- Zheng J, Wen D, Weng L. Effects of Yishen Jiangzhuo Granule on the bone metabolism of patients with stage 3-4 chronic kidney disease and its correlation with the immune indices. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012;32:183-7. [PubMed]
- Meng L, Van Putten V, Qu L, et al. Altered expression of genes profiles modulated by a combination of Astragali Radix and Angelicae Sinensis Radix in obstructed rat kidney. Planta Med 2010;76:1431-8. [Crossref] [PubMed]
- Aronov PA, Luo FJ, Plummer NS, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol 2011;22:1769-76. [Crossref] [PubMed]
- Zhu B, Lin Y, Zhu CF, et al. Emodin inhibits extracellular matrix synthesis by suppressing p38 and ERK1/2 pathways in TGF-β1-stimulated NRK-49F cells. Mol Med Rep 2011;4:505-9. [PubMed]
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17-28. [Crossref] [PubMed]
- McAlister FA, Ezekowitz J, Tonelli M, et al. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004;109:1004-9. [Crossref] [PubMed]
- Butler J, Chirovsky D, Phatak H, et al. Renal function, health outcomes, and resource utilization in acute heart failure: a systematic review. Circ Heart Fail 2010;3:726-45. [Crossref] [PubMed]
- Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010;122:265-72. [Crossref] [PubMed]