
Rare paraneoplastic erythroderma associated with ectopic neuron-specific enolase deposition in basal cells
Introduction
Erythroderma refers to an extreme state of skin erythema and scaling, covering more than 90% of the body surface (1). Several causes may result in the presentation of erythroderma, including drug reactions, malignancies, systematic diseases, infections and idiopathic disorders (2). Largely, erythroderma was associated with malignancies, such as T cell lymphoma, gastric cancer, lung cancer and hepatocellular cancer (3-5). In cases of paraneoplastic erythroderma, the cutaneous finding may occur as the only symptom of a malignancy. Thus, the disease with a sudden erythroderma without any known cause can be considered as the malignancy (6).
Although many cases associated with malignancies have been reported, the pathogenesis of cancer related erythroderma is still unclear. Herein we present a patient with severe erythroderma and contemporary large cell neuroendocrine carcinoma (LCNEC) of the lung. The cutaneous lesions of patient recovered completely after resection of cancer. Further research strongly supported that ectopic neuron-specific enolase (NSE) deposition might result in the paraneoplastic erythroderma. To our knowledge, this is the first case that illustrated the relationship between paraneoplastic erythroderma and NSE.
Patients and methods
A 68-year-old Chinese man presented with a 1-month history of skin erythema and scaling in almost all over the body (Figure 1A). In addition, he had a 2-month history of stethalgia and discontinuous cough. Past medical history was unremarkable and social history was positive for 60 pack-years smoking. The patient was referred to his dermatologist with extensive skin erythema and scaling. Chest computer tomography (CT) showed an upper lobe mass of the left lung (Figure 1B). The left upper lobectomy with mediastinal lymphadenectomy in video-assisted thoracoscopic surgery was performed. Histology of cancer demonstrated poorly differentiated LCNEC of the lung (Figure 1C) with no evidence of cancer metastasis in the hilar or mediastinal lymph nodes (T2N0M0, stage IB). Three weeks after resection, the whole skin of the patient recovered completely without any erythema and scaling (Figure 1D). The study was approved by the Institutional Ethics Committee of the Fourth Military Medical University, and all patients volunteered to participate in the study and signed informed consent forms.
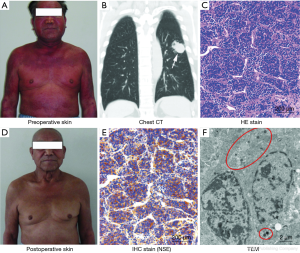
Immunohistochemistry (IHC) stain was performed in primary cancer, cancer surrounding tissues, perioperative skin, postoperative skin tissues and normal skin tissues from five healthy men, involving neuroendocrine markers (neuropeptide Y, serotonin, calcitonin gene-related peptide, NSE, chromogranin A, synaptophysin), epithelial markers (CK7, CK20) and first line hematolymphoid markers (CD10, CD20, CD99, CD117 and myeloperoxidase). NSE showed 2+ positivity in primary cancer tissues (Figure 1E). CD99 and CD117 were also observed as 1+ staining in primary cancer tissues, whereas all the neuroendocrine markers were negative in cancer surrounding lung tissue. Many round vacuoles with a diameter of 100–200 nanometer (nm) were found in the cytoplasm by using transmission electron microscope (TEM) (Figure 1F). These round vacuoles had the characteristic of neuroendocrine carcinoma, containing too much neurotransmitter. In this case, NSE was inferred to the main neurotransmitter depending on IHC staining.
As compared with skin tissues of five healthy men, a chronic perivascular inflammatory infiltrate, acantholysis and parakeratosis were the prominent histopathologic feature in perioperative skin (Figure 2A). Further IHC revealed positive stains of NSE (2+) (Figure 2B), CD117 (1+) and CD99 (1+) in basal cells, yet other neuroendocrine markers, epithelial markers and first line hematolymphoid markers were negative. TEM supplied ultrastructural changes in the process of erythroderma recovery. In perioperative skin, we found the acantholysis and parakeratosis occurred on the top of basal layer where the connection between prickle cells became looser, the intercellular space between desmosomes became wider, and the tonofilaments disappeared (Figure 2C,D). The morphologic changes of the basal cells showed comprehensive atrophy and thin cytoplasm, as well as a few round vacuoles (Figure 2E). However, it was difficult to identify the compounds of these extra round vacuoles.
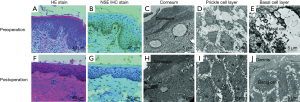
Three months after surgery, postoperative skin biopsy presented nearly normal skin tissues, accompanied with a negative stain of NSE, but positivity of CD99 (1+) in basal cells (Figure 2F,G). In the postoperative skin tissues, the corneum and prickle cells grew compactly, and the intercellular space became narrow, and the tonofilaments were revived (Figure 2H,I,J). Otherwise, serum tumor markers including carcinoembryonic antigen, alpha fetoprotein, carbohydrate antigen 199, carbohydrate antigen 125, neuropeptide Y, serotonin, calcitonin gene-related peptide, NSE, chromogranin A and synaptophysin were tested by radioimmunoassay. Serum NSE level declined from 105 to 25 µg/mL before and after surgery. Other markers were ranged in normal levels.
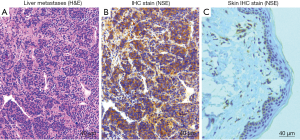
Nine months after surgery, the patient suffered skin erythema and scaling on his whole-body surface again. Abdominal CT and brain MRI suggested solitary liver metastases and diffuse brain metastases. Liver paracentesis guiding by ultrasound was conducted, and the pathology of biopsy tissues illustrated the LCNEC (Figure 3A) and 3+ positive stain of NSE (Figure 3B). CD99 was also observed as 1+ positive stain in cancer tissues. The skin biopsy tissues showed 2+ positive stain of NSE (Figure 3C). Serum NSE increased to 119 µg/mL. The serum leukocyte, especially neutrophils and monocyte, increased to 2-fold of normal values. The patient received chemotherapy treatment with cisplatin (75 mg/m2, every 21 days) and docetaxel (75 mg/m2, every 21 days) twice, and radiation therapy (60 Gy) for brain metastases. Evaluation after two cycles of chemotherapy showed marked improvement in erythroderma and reduction of tumor volume. However, chemotherapy was terminated due to severe myelosuppression. The patient experienced recurrent worsening of erythroderma and onset of peripheral oedema. Serum NSE further increased to 199 µg/mL. Thus, the patient received palliative care. Eleven months after surgery, the patient died of cancer cachexia and multiple organ failure.
Discussion
The onset of erythroderma is usually gradual and insidious, except in drug-induced cases (2). Paraneoplastic erythroderma is a rare disease in clinical practice. Most of the tumors companied with skin symptoms, such as granuloma fungoides and Hodgkin’s lymphoma, originate from bone marrow or lymph node. A report (7) showed that the paraneoplastic erythroderma was related with the tumor secretion, including cytokines (IL-1, IL-2, IL-8) and cell adhesion factors (vcam-1, ICAM-1). The interaction of tumor secretion can promote the enrichment of lymphocyte and monocyte. However, few paraneoplastic erythrodermas are caused by lung cancer up to date. In this study, the severe skin erythema and scaling had appeared for 1 month before the upper lobe mass of left lung was discovered, and the cutaneous lesion was completely resolved after surgery. However, the skin erythema and scaling occurred again when the cancer recurred in the liver and brain. Paraneoplastic syndrome was characterized by skin lesions that progress despite therapy, were resistant to standard treatment, and responded promptly to the treatment of tumor (6). Thus, the parallel course between the carcinoma and cutaneous lesion strongly supported the diagnosis of paraneoplastic syndrome.
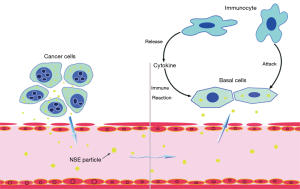
Cancer induced autoimmune reaction and cytokines might be related to the pathogenesis, which is still lack of robust evidence (8). The precise mechanisms underlying these paraneoplastic erythrodermas remain unclear, but some views are thought to be toxic or allergic reactions caused by unknown factors (9). In this study, NSE may be a critical intermediate between cancer and erythroderma. Strong expression of NSE was detected in primary and recurrent LCNEC which was a classic category of pulmonary neuroendocrine tumors. High level of serum NSE secreted from the cancer represented as the tumor activity and therapeutic effect. Furthermore, strong expression of NSE was found in the skin basal cells which had rare expression of these neuroendocrine markers (9). The deposition of NSE in basal cells as well as erythroderma was alleviated following the surgery, but both of them worsened after recurrence of the cancer. Thus, the deposited NSE in skin basal cells might stimulate a direct autoimmune reaction, which might be a crucial pathogenic factor of erythroderma (see Figure S1).
Acknowledgements
The authors thank Professor Jiayan Liu at the Department of Pathology, Xijing Hospital, the Fourth Military Medical University, China for the help with pathological diagnosis. This work was supported by the Basal Research Innovate Fund and Young Talents Fund of Tangdu Hospital, the Fourth Military Medical University, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from all patients for publication of this manuscript and any accompanying images.
References
- Holden CA, Breth-Jones J. Eczema, Lichenification, Prurigo and Erythroderma. In: Burns T, Breathnach S, Cox N, et al. editors. Rook's Textbook of Dermatology, 7th Edition. Oxford: Blackwell scientific, 2004;1-17.
- Botella-Estrada R, Sanmartín O, Oliver V, et al. Erythroderma. A clinicopathological study of 56 cases. Arch Dermatol 1994;130:1503-7. [Crossref] [PubMed]
- Akhyani M, Ghodsi ZS, Toosi S, et al. Erythroderma: a clinical study of 97 cases. BMC Dermatol 2005;5:5. [Crossref] [PubMed]
- Kim SW, Kang YS, Park SH, et al. A case of erythrodermic dermatomyositis associated with gastric cancer. Ann Dermatol 2009;21:435-9. [Crossref] [PubMed]
- Khaled A, Sellami A, Fazaa B, et al. Acquired erythroderma in adults: a clinical and prognostic study. J Eur Acad Dermatol Venereol 2010;24:781-8. [Crossref] [PubMed]
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008;7:327-40. [Crossref] [PubMed]
- Pileri A, Pellegrini C, Agostinelli C, et al. Erythroderma and non-Hodgkin T-cell lymphoma: what else, apart from Mycosis Fungoides and Sézary syndrome?. Eur J Dermatol 2017;27:49-53. [PubMed]
- Nagler AR, Samimi S, Schaffer A, et al. Peripheral blood findings in erythrodermic patients: importance for the differential diagnosis of Sézary syndrome. J Am Acad Dermatol 2012;66:503-8. [Crossref] [PubMed]
- Hanafusa T, Igawa K, Takagawa S, et al. Erythroderma as a paraneoplastic cutaneous disorder in systemic anaplastic large cell lymphoma. J Eur Acad Dermatol Venereol 2012;26:710-3. [Crossref] [PubMed]


