
Can the echocardiographic LV mass equation reliably demonstrate stable LV mass following acute change in LV load?
Introduction
Cardiac remodeling is defined as ‘alterations in size, geometry, shape, composition and function of the heart resulting from cardiac load or injury’ (1). When the left ventricle (LV) faces pressure or volume overload, cellular hypertrophy ensues, increasing overall LV mass and reducing the stress on myocardial wall (2-4). It is well known that LV hypertrophy and increased LV mass are associated with poor prognosis (5-8). Since LV mass is most commonly assessed using echocardiography, it is important to understand the accuracy of the technique and the ability of the assessment to measure changes in LV mass over time.
Devereux first validated the use of LV mass measurement using echocardiography in 34 pre-mortem subjects compared to LV weight on autopsy (9). This equation continues to be employed in widespread use, with slight modifications, despite few other confirmatory studies of its validity. The American Society of Echocardiography advocates the using version of the Devereux equation:
LV mass = 0.8 × {1.04[(LVID + PWT + SWT)3 − (LVID)3]} + 0.6
To calculate the LV mass, where LVID is the LV internal diameter in diastole, PWT is the posterior wall thickness in diastole, and SWT is the septal wall thickness in diastole (10). Since the LV mass equation relies on measurements of LV wall thickness and LV chamber diameter, LV wall thickness may therefore be expressed as a function of LV diameter:
LV wall thickness = {[(LV mass – 0.6)/(1.04×0.8)] + LVID3]1/3 – LVID}/2
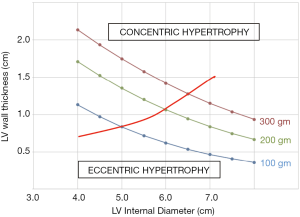
Where LV wall thickness is the average of PWT and SWT. This relationship is shown in Figure 1 for subjects with three different LV masses. This equation demonstrates that if LV mass remains constant, a decrease in LV diameter is necessarily met with an increase in LV wall thickness. We aimed to test the LV mass equation’s validity by measuring the LV mass before and after an acute change in LV loading. We chose to do this in the setting of aortic valve replacement for regurgitation, since there is a large change in the pressure and volume load on the LV. We assumed that LV mass would be unlikely to change in just a few days after surgery (11-13). We therefore anticipated that a decrease in LV diameter, known to be associated with this procedure, would be accompanied by an increase in LV wall thickness since this would be necessary for LV mass to remain unchanged.
Methods
Study population
The cardiology database Apollo (Lumedx, Oakland, CA) was queried for aortic valve replacement (AVR) performed at Montefiore Medical Center from July 2007 to January 2017. Only patients undergoing AVR for aortic regurgitation (AR) were included in the study.
Patients were excluded if they were less than 18 years, had poor echocardiographic image quality or had co-existing severe valve disease [i.e., aortic stenosis (AS), mitral stenosis (MS) or mitral regurgitation (MR)]. Patients were also excluded if they had prior valve replacement or were lacking post-operative echocardiograms in 2–15 days following the procedure.
We also included a control group comprised of 14 subjects who underwent isolated coronary artery bypass grafting (CABG) without valve surgery (2:1 ratio to AR subjects). The control group was studied to see if changes in LV diameter and wall thickness would be seen after cardiac surgeries when large changes in LV loading were not expected to occur. The controls were selected from the same database as the AR subjects. They were selected from a similar time frame and were matched to the AVR subjects based on age, sex and body mass index (BMI).
Clinical data were obtained from retrospective chart review. The Institutional Review Board of Albert Einstein College of Medicine approved this study (IRB #: 2017-7596).
Echocardiographic variables
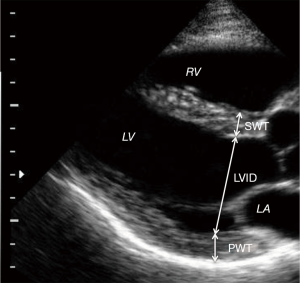
All of the selected pre-operative and post-operative echocardiograms had been obtained using Phillips IE-33. Images had been archived on a long-term storage server (Centricity; General Electric), but the images were retrieved and analyzed using Xcelera (Philips). All images were re-analyzed by two independent readers. Reader1 (R1) is a physician who was trained specifically to make echocardiographic measurements for this study. Reader2 (R2) is a level III, high volume reader of echocardiograms at a busy city hospital. Inter-observer variability was tested between our two investigators and also compared to the clinical echocardiogram reports. LV wall thickness and diameter were measured at end diastole as shown in Figure 2.
Statistical analysis
Statistical analysis was performed using STATA (College Station, TX). Normally distributed data were presented as mean ± standard deviation. Comparison of means was performed using 2-sample t-test. Comparison of categorical data was performed using Chi-squared test. The P were considered to be statistically significant if <0.05. The null hypothesis was that LV mass is unchanged between the pre-operative and post-operative echocardiograms. LV mass (pre) – LV mass (post) =0. The paired t-test was used to compare the pre-operative and post-operative measurements including LV mass, LVID, SWT and PWT.
Results
Patient characteristics and surgical features
Our study group consisted of 28 adult patients who underwent AVR for primary AR. They were chosen from 144 patients who underwent AVR during the study period. We had excluded 86 due to lack of echocardiograms in the specified time frame, 15 due to history of prior prosthetic valve placement, 11 due to presence of severe MS or MR. Two due to poor image quality and 2 due to being younger than 18 years. The control group of 14 subjects was selected from 152 patients that underwent isolated CABG. Subjects were selected that matched the study group for age, sex and BMI.
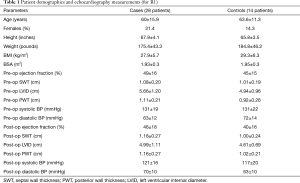
The baseline characteristics of cases and controls are listed in Table 1. Compared to the controls, the study subjects had lower body mass index (BMI) and had increased LVID and PWT on the preoperative exams. Otherwise, the groups were similar.
Full table
The most common etiologies of AR were fibro-calcific valve disease (n=11) and infective endocarditis (n=7). 19 patients in AVR group underwent only AVR and 9 patients underwent both AVR and CABG. The prosthetic valves were either bioprosthetic (n=22) or mechanical (n=6). All surgeries were performed via sternotomy (i.e., none were transcutaneous). Aortic regurgitation was thought to be chronic in 21 subjects and acute in 7. For AVR group, echocardiograms were acquired a median 10 [range, 0–128] days preoperatively and a median 7 [range, 2–15] days postoperatively. The average pre-operative LV mass index (LVMI) was 110 [range, 67–226] g/m2 for women and 131 [range, 63–341] g/m2 for men. No aortic valve regurgitation was seen on any of the postoperative echocardiograms.
Echocardiographic measurements and LV mass calculation
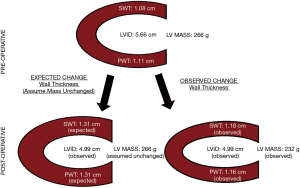
Using measurements made by the main study reader, LVID decreased postoperatively from 5.7±1.2 to 4.9±1.0 cm, P<0.001. SWT was noted to increase from 1.08±0.20 to 1.18±0.27 cm, P=0.03, but PWT was unchanged 1.11±0.21 to 1.16±0.27 cm, P=0.21. Accordingly, the LV mass equation calculated a decrease in LV mass from 266±126 to 232±99 gm, P=0.01, Figure 3.
Measurements made on the clinical echo reports and by a second study reader also showed a decrease in LVID postoperatively. Measurements of the SWT and PWT on the clinical echocardiographic reports were not significantly changed from preoperative values. Accordingly, the LV mass equation calculated a decrease in LV mass postoperatively. Using summary data for all echocardiographic readers (the study readers and the clinical echocardiographic reports), no change was seen for LVID, SWT and PWT measurements in the control group.
Inter-observer agreement (pre-operative measurements)
In the AVR group, no significant bias was detected between readers R1 and R2 for the measurement of SWT, LVID, PWT and calculated LV mass, Figure 4, Table 2. In the CABG-only group, no significant bias was detected between R1 and R2 for the measurement of preoperative SWT, LVID and calculated LV mass. A small bias (1.00 mm, P=0.001) was detected between R1 and R2 for the measurement of PWT in the CABG-only group.

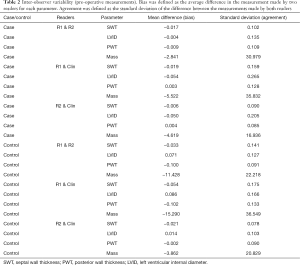
Full table
In the AVR group, agreement between R1 and R2 (i.e., the standard deviation of the difference in reader measurements) was typically 1.0 mm for SWT, 1.4 mm for LVID and 1.1 mm for PWT, Figure 4. In other words, the readers agreed within these limits 68% of the time (one standard deviation). In the CABG-only group, agreement between R1 and R2 was typically 1.4 mm for SWT, 1.3 mm for LVID and 0.9 mm for PWT. In AVR group, intraclass correlation coefficient (ICC) for R1 and R2 was 0.90 for SWT, 0.99 for LVID and 0.88 for PWT. In CABG-only group, ICC for R1 and R2 was 0.78 for SWT, 0.99 for LVID and 0.95 for PWT.
Intra-observer agreement (postoperative change)
In the AVR group, agreement for R2 between initial and repeat measurements was typically 0.11 mm for SWT, 0.14 mm for LVID and 0.11 mm for PWT. In CABG-only group, agreement for R2 between initial and repeat measurements was 0.18 mm for SWT, 0.18 mm for LVID and 0.11 mm for PWT. In AVR group, ICC for R2 between initial and repeat measurements was 0.97 for SWT, 0.99 for LVID and 0.97 for PWT. In CABG-only group, ICC for R2 between initial and repeat measurements was 0.96 for SWT, 0.99 for LVID and 0.99 for PWT.
Discussion
As expected, following AVR for AR, we found that LV diameter decreased. While one of our readers found a small concomitant increase in SWT, overall we did not find an increase in LV wall thickness that would be expected for LV mass calculation to remain constant. Therefore, the LV mass equation calculated that LV mass decreased after the valve replacement. There are several possible explanations for our findings. One explanation is that LV mass really did decrease in the immediate postoperative period. While this explanation is somewhat plausible, we feel that this is not likely. Animal studies that have evaluated the development of cellular hypertrophy suggest this process probably takes a minimum of several weeks (11). We presume that regression of cellular hypertrophy and LV mass should take at least as long. Other studies have looked at the rate of LV mass regression in various contexts. However, these works have relied on the Devereux equation or its variants using either echocardiography or MRI (14-17), and cannot therefore be relied upon to confirm the rate of LV mass change.
We contend that measurement error in the assessment of LV wall thickness is likely to hinder assessment of change in LV mass. Transthoracic echocardiography is a relatively low-resolution technique, quality of acoustic windows and operator experience largely determine the accuracy of determination of interfaces for LVID and wall thickness. Minute errors performed in these linear measurements are amplified when they are cubed before being utilized in the LV mass equation. We found inter-observer variability in wall thickness in excess of 10% between our study readers, which is well within the reported variability from prior literature (18). While errors in measurement are probably random (i.e., not systematic error), such level of error is probably large enough to prevent detection of increased wall thickness on the postoperative echocardiograms. As shown in Figure 3, a measurement error of only 1 mm could account for our findings that calculated LV mass decreased postoperatively. Rychik et al. (19) were able to demonstrate that decreased LV diameter was met with increased LV wall thickness in pediatric subjects undergoing Fontan procedure. In their study, LV mass remained constant one week postoperatively. We presume that LV wall thickness may have been easier to measure with greater precision in pediatric subjects due to their small body habitus.
There may also be a geometric explanation for our observed decrease in calculated LV mass postoperatively. The ASE LV mass equation relies on linear measurements that are performed in the basal LV on the assumption that mass is uniformly distributed throughout the ventricle. However, with acute change in loading conditions, there is a possibility that there is a change in the distribution of LV mass away from the base toward the more apical segments.
Although widely used in routine day-to-day clinical practice, the accuracy and precision of LV mass equation has been questioned, especially when used in geometrically distorted ventricles.
The original equation has shown a good correlation between true LV mass and autopsy studies, which is the gold standard for LV mass estimation (9). However, in the initial study, none of the 34 patients had severely distorted left ventricle geometry (9,20,21). It was essentially created for ‘normal hearts’ and did not include patients with extremes of LV mass or dilation. Devereux et al., in another study (20) included 55 patients, some of who had distorted LV geometry (20 patients with MI, 14 with hypertension, 17 with valvular heart disease, 8 with heart failure) and noted a higher degree of error in estimating LV masses. Woythaler et al. (21) and Reichek et al. (22) also demonstrated a considerable overestimation of LV mass by M-mode echocardiography when patients with deranged LV geometry were included.
Concerns about the utilization of this equation were raised as early as 1983 when patients with ESRD demonstrated a significant change in LVID over the course of a single hemodialysis session (23). A subsequent prospective, blinded study of 15 ESRD patients (12) demonstrated a significant decrease in LV mass index (LVMI), again over a single hemodialysis session. This was primarily driven by a decrease in LVID without a significant increase in wall thickness. It is self-apparent that a ‘true change’ in LV mass index should not occur in a span of hours. This raised serious concerns about its use in longitudinal studies, especially with acutely changing loading conditions. In recent years, several studies have used the LV mass equation to demonstrate decrease in LV mass in the immediate post-operative period following acute unloading of dilated ventricles, with LVAD implantation (24) or AVR (14-17). Given our findings, it is critical that such interpretations be made with caution.
Study limitations
Our study had several limitations. Our sample size was small with 28 patients. We excluded patients that did not have post-operative echocardiograms within 2–15 days of valve replacement. This may have included patients who died, transferred to another facility or stopped following at our center. This may have introduced a selection bias.
Conclusions
While the expected decrease in LVID was observed after AVR for AR, measured values for the postoperative SWT and PWT did not increase as much as would be predicted by the ASE mass equation to keep LV mass unchanged. Accordingly, the ASE mass equation reported a reduction in LV mass immediately after AVR. Since a true change in LV mass in the immediate period after AVR is unlikely, we feel these results highlight an important limitation of the mass equation when used in dilated ventricles with acute change in loading conditions. Caution should therefore be used when using this equation to document serial changes in LV mass over time.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Clinical data were obtained from retrospective chart review. The Institutional Review Board of Albert Einstein College of Medicine approved this study (IRB #: 2017-7596).
References
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35:569-82. [Crossref] [PubMed]
- Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol 1960;5:370-82. [Crossref] [PubMed]
- Sasayama S, Ross J Jr, Franklin D, et al. Adaptations of the left ventricle to chronic pressure overload. Circ Res 1976;38:172-8. [Crossref] [PubMed]
- Ross J Jr. Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog Cardiovasc Dis 1976;18:255-64. [Crossref] [PubMed]
- Verdecchia P, Carini G, Circo A, et al. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001;38:1829-35. [Crossref] [PubMed]
- Schillaci G, Verdecchia P, Porcellati C, et al. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000;35:580-6. [Crossref] [PubMed]
- Ghali JK, Liao Y, Simmons B, et al. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med 1992;117:831-6. [Crossref] [PubMed]
- Verma A, Meris A, Skali H, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging 2008;1:582-91. [Crossref] [PubMed]
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977;55:613-8. [Crossref] [PubMed]
- Nucifora G, Tantiongco JP, Crouch G, et al. Changes of left ventricular mechanics after trans-catheter aortic valve implantation and surgical aortic valve replacement for severe aortic stenosis: A tissue-tracking cardiac magnetic resonance study. Int J Cardiol 2017;228:184-90. [Crossref] [PubMed]
- Du Y, Plante E, Janicki JS, et al. Temporal evaluation of cardiac myocyte hypertrophy and hyperplasia in male rats secondary to chronic volume overload. Am J Pathol 2010;177:1155-63. [Crossref] [PubMed]
- Harnett JD, Murphy B, Collingwood P, et al. The reliability and validity of echocardiographic measurement of left ventricular mass index in hemodialysis patients. Nephron 1993;65:212-4. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Shin S, Park PW, Han WS, et al. Mass reduction and functional improvement of the left ventricle after aortic valve replacement for degenerative aortic stenosis. Korean J Thorac Cardiovasc Surg 2011;44:399-405. [Crossref] [PubMed]
- Jung SH, Lee JW, Je HG, et al. Surgical outcomes and post-operative changes in patients with significant aortic stenosis and severe left ventricle dysfunction. J Korean Med Sci 2009;24:812-7. [Crossref] [PubMed]
- Ahmad N, Shahbaz A, Ghaffar A, et al. Early left ventricular remodeling after aortic valve replacement. J Ayub Med Coll Abbottabad 2007;19:10-4. [PubMed]
- Petrov G, Regitz-Zagrosek V, Lehmkuhl E, et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 2010;122:S23-8. [Crossref] [PubMed]
- Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation 1995;91:1739-48. [Crossref] [PubMed]
- Rychik J, Jacobs ML, Norwood WI Jr. Acute changes in left ventricular geometry after volume reduction operation. Ann Thorac Surg 1995;60:1267-73; discussion 1274. [Crossref] [PubMed]
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450-8. [Crossref] [PubMed]
- Woythaler JN, Singer SL, Kwan OL, et al. Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol 1983;2:305-11. [Crossref] [PubMed]
- Reichek N, Helak J, Plappert T, et al. Anatomic validation of left ventricular mass estimates from clinical two-dimensional echocardiography: initial results. Circulation 1983;67:348-52. [Crossref] [PubMed]
- Nixon JV, Mitchell JH, McPhaul JJ Jr, et al. Effect of hemodialysis on left ventricular function. Dissociation of changes in filling volume and in contractile state. J Clin Invest 1983;71:377-84. [Crossref] [PubMed]
- Drakos SG, Wever-Pinzon O, Selzman CH, et al. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol 2013;61:1985-94. [Crossref] [PubMed]


