
Non-intubated thoracoscopic surgery for lung cancer in patients with impaired pulmonary function
Introduction
Lung cancer is a leading cause of death worldwide, as well as in Taiwan (1,2). With low-dose computed tomography screening, it is possible to detect lung cancer at an earlier stage (3). The best treatment for early-stage non-small cell lung cancer (NSCLC) is surgery. However, pulmonary resection of lung cancers in patients with impaired lung function is risky and controversial (4,5).
In past decades, video-assisted thoracic surgery (VATS) has been well demonstrated as an effective and safe surgical approach for various thoracic diseases and is currently considered the treatment of choice to shorten the length of hospital stay (6). VATS lung resections have been successfully carried out in patients with impaired lung function (7-9). For VATS, intubated general anesthesia with one-lung ventilation has widely been regarded mandatory. Nonetheless, complications after intubated general anesthesia are not negligible (10-12). Therefore, non-intubated VATS has emerged as an alternative in a variety of thoracic procedures with encouraging results (13,14).
Non-intubated VATS is enthusiastically used for lung volume reduction surgery in patients with severe emphysema (14). It is unknown whether if non-intubated VATS may be successfully adapted to lung cancer surgery in patients with pulmonary insufficiency. In this study, we reviewed our 7-year experience with non-intubated VATS for lung cancer surgery in patients with impaired pulmonary function.
Methods
This retrospective study was reviewed and approved by the Research Ethics Committee of National Taiwan University Hospital (approval No.: 201505004RINA). All patients gave their consent to a non-intubated technique after an explanation of the anesthesia and surgical procedures before undergoing surgery.
Patients
We began performing non-intubated VATS for the management of lung tumors since 2009. Therefore, a prospectively maintained database of all patients undergoing non-intubated VATS was used to identify patients with impaired lung function that underwent resections for lung tumors. Patients considered for non-intubated VATS were selected by both the attending surgeons and anesthesiologists after reviewing each patient’s medical records and discussing the procedures with the patients. In this study, the inclusion criteria comprised patients with impaired lung function [defined as a preoperative forced expiratory volume in one second (FEV1) below 70% of predicted] (15), with an American Society of Anesthesiologists physical status score of 4 or less and a lung tumor less than 6 cm in the outer half of the lung. Patients with anticipated difficult airway management, chest wall or spinal deformity, bleeding disorders, body mass index (BMI) =30 kg/m2 or greater, or evidence of pleural adhesions on computed tomography of the chest were excluded.
Anesthetic setting, induction, and maintenance
The anesthesia management for non-intubated VATS has been described previously (16). In summary, all patients were pre-medicated with intravenous fentanyl (50–100 µg) and glycopyrrolate (0.2 mg) as an antisialagogue. Standard monitoring included pulse oximetry, electrocardiography, and arterial blood pressure. A detector placed into one nostril was used to monitor end-tidal carbon dioxide continuously and respiratory rate. A bispectral index (BIS) sensor (BIS Quatro, Aspect Medical System, Norwood, MA, USA) was applied to the forehead to monitor the depth of sedation. The patients were induced with intravenous propofol using a target-controlled infusion method to maintain their sedation at a Ramsay sedation score of III (responsive to verbal commands only) or in a BIS range of 40 to 60. Additionally, incremental intravenous injections of fentanyl (25 µg) were given to maintain a respiration rate between 12 and 20 breaths/min. During the whole procedure, the patients were placed in the lateral decubitus position while spontaneously breathing oxygen through a ventilation mask, maintaining oxygen saturation greater than 90%.
Loco-regional anesthesia was achieved through either a thoracic epidural catheter or intercostal nerve blockade. Thoracic epidural anesthesia was performed by insertion of an epidural catheter at the T5/T6 or T6/T7 thoracic interspace to achieve a sensory block between the T2 and T9 dermatomes. Anesthesia was then maintained by continuous infusion of 2% lidocaine. Internal intercostal nerve and vagal blockades were produced under thoracoscopic guidance.
Surgical technique for non-intubated VATS
We used a three-port approach to performing thoracoscopic surgery (16). Briefly, a 1.5-cm incision for thoracoscopic instrumentation was first created after local infiltration of 2% lidocaine in the seventh or eighth intercostal space in the mid-axillary line. Another working port and a utility port (2–5-cm incision) were made in the sixth intercostal space in the auscultatory triangle and anteriorly at the fifth intercostal space, respectively. We used Alexis wound protectors (Applied Medical, Santa Margarita, CA, USA) to cover the incisions and keep them open. The operated lung collapsed gradually by spontaneous breathing of the patients after creating an iatrogenic pneumothorax by thoracoscopic incisions. During the surgery, the collapse of the operated lung was satisfactory for many types of procedures.
The intercostal and vagal nerves were blocked using thoracoscopic guidance (16). The third to the eighth intercostal nerves under the parietal pleura, 2 cm lateral the sympathetic chain, were blocked using a 25-guage top-winged infusion needle by infiltration with 0.5% bupivacaine (1.5 mL in each intercostal space). An intrathoracic vagal block was produced by infiltration of 0.5% bupivacaine (3 mL) near the vagus nerve at the level of the aortopulmonary window for left-sided procedures or at the level of the lower trachea for right-sided procedures. This vagal block effectively inhibited the cough reflex for 3 hours or longer during thoracoscopic manipulation.
The surgical techniques used for thoracoscopic wedge resection, segmentectomy, lobectomy, and mediastinal lymph node dissection are described elsewhere (17-19). For thoracoscopic lobectomy and segmentectomy, pulmonary vessels, bronchi, and incomplete fissures were divided using an endoscopic stapling device. Assisted positive pressure ventilation via a face mask was applied to check the definite location of the resected bronchus after temporarily clamping the targeted bronchus. For thoracoscopic wedge resection, endoscopic stapling was applied for partial lung resection, including the tumor. The resected specimen was removed in an organ retrieval bag through the utility incision. Use of retractor and spreading and cutting ribs were avoided in all patients. Staple line reinforcement device (Endo GIA™ Ultra, Covidien/Medtronic, CA, USA) was used for prevention of air leakage; otherwise, 4-0 prolene was used to ensure the suture of the pleural surface of the lung. The collapsed lung was then recruited through a manually assisted mask ventilation and checked for air leakage through thoracoscopic observation. After the operation, a 28-French chest tube was inserted through the lowest incision.
During wound closure and chest tube insertion, propofol infusion was discontinued. After the patient was fully awake, they were asked to breathe deeply and cough to re-expand the collapsed lung.
Conversion to tracheal intubation
Indications for conversion from non-intubated anesthesia to intubated one-lung ventilation included profound respiratory movement, massive pleural adhesions, refractory hypoxemia (oxygen saturation on pulse oximetry of less than 85% for more than 5 minutes), severe hypercapnia, unstable hemodynamic status or uncontrolled bleeding requiring an emergency thoracotomy. For intubation, the surgical wound was sealed with a transparent waterproof dressing (Tegaderm Film, 3M Health Care, Neuss, Germany) after temporary insertion of a chest tube to re-expand the collapsed lung. The trachea was then intubated using a single lumen endotracheal tube under bronchoscopic guidance, followed by lung separation using an endobronchial blocker. A change in the patient’s position was not necessary.
Postoperative analgesics and care
Postoperative analgesia was provided either by patient-controlled analgesia with epidural infusion of 0.1% bupivacaine and fentanyl (1.25 µg/mL) or with intravenous morphine (1 mg/mL) for 2–3 days if patients requested. In addition, oral non-steroidal analgesics and acetaminophen were also given once patients resumed oral intake 2–4 hours after surgery. Postoperative pain intensity was evaluated using a numeric pain intensity scale where 0 represented no pain and 10 represented intractable pain. Patients underwent chest radiography the next morning. If no air leak was present, and drainage was <200 mL in a 24-hour period, the chest tube was removed. An air leak was defined as prolonged if it lasted longer than 3 days. All postoperative complications were recorded.
Data collection and analyses
The clinical data, anesthesia results, operative findings and outcomes, adverse effects and complications, and pathology of the nodules were collected from the medical records. Arterial blood gases were determined before skin incision (preoperative, baseline), 20 minutes after lung collapse commenced (intraoperative, spontaneous breathing during open surgical pneumothorax, OSP), and after wound closure (postoperative, two-lung ventilation resumed). Anesthetic complications were defined by the patients’ complaints or complications caused by the anesthetic procedures. Operative complications were complications caused by the operation procedures. The pain intensity data were collected from the nursing records. Descriptive statistics were reported as median (range) or mean ± standard deviation for continuous data, and as number (%) for categorical data.
Results
From August 2009 to June 2015, we performed 779 cases of non-intubated VATS. Of them, 30 patients had FEV1 <70% of predicted. There were 28 patients undergoing primary lung tumor resections. Table 1 summarizes the clinical characteristics of the cohort. The median patient age was 70.8 years, and 9 (32%) were women. The mean FEV1 of the patients was 57.9% of the predicted value. The most common causes of impaired lung function were a history of heavy smoking and comorbid chronic obstructive pulmonary disease (COPD).
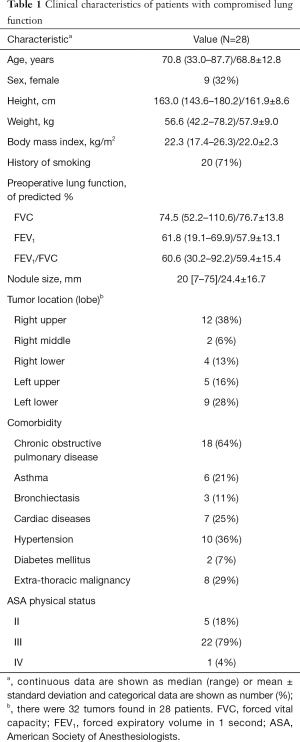
Full table
Table 2 provides the operative and anesthesia results. For loco-regional anesthesia, 18 patients used thoracic epidural anesthesia and 10 patients used intercostal nerve blocks. Sub-lobar or lobar pulmonary resection was performed with mediastinal lymph node dissection only for patients with primary lung cancer, in accordance with the pathologic diagnoses of the tumors (Table 3). The median duration of anesthesia induction was 20 minutes. The median surgical duration ranged from 76 minutes for wedge resection only to 180 minutes for lobectomy with lymphadenectomy (Table 2).
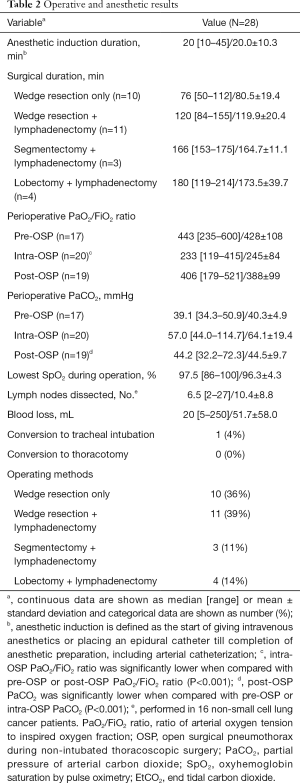
Full table
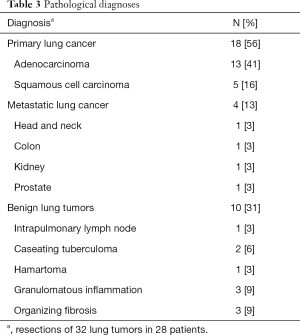
Full table
Throughout surgery, the oxygen saturation measured by pulse oximetry was satisfactory. The mean ratio of arterial oxygen tension to inspired oxygen fraction (PaO2/FiO2) during intra-OSP was significantly lower compared with the pre- and post-OSP PaO2/FiO2 ratio (P<0.001). The median highest partial pressure of arterial carbon dioxide during OSP was 57.0 mmHg. Conversion to tracheal intubation and one-lung ventilation was required in one patient, an 80-year-old man with a BMI of 26.3 kg/m2, because of persistent intraoperative wheezing and labored breathing. The intubation was performed smoothly in the lateral position. The patient required ventilator support postoperatively, and the ventilator was weaned off the next morning. No patients required conversion to a thoracotomy or blood component therapy.
Table 4 provides the treatment outcomes. Complications of anesthesia developed in only 1 patient who had a mild sore throat and dizziness after awakening. The postoperative pain intensity scores were very low on the first and second postoperative days. The median duration of postoperative chest tube drainage was 3 days. Of the 28 patients, 19 patients (68%) stayed in the intensive care unit for a median of 1 night, and the median postoperative hospital stay was 6 days. Prolonged hospital stays were mostly caused by persistent air leaks requiring chemical pleurodesis or low-pressure suction (5 patients, 18%) (Table 5). No deaths or major complications occurred. Four patients (14%) were readmitted within 30 days because of wound dehiscence, pleural effusion, pneumothorax, or urinary tract infection requiring further treatment. One patient died from urosepsis complicated with septic shock one month after surgery, suspect metastatic colon cancer related. Six months after surgery, 27 of 28 patients were alive without complications due to non-intubated thoracoscopic surgery.
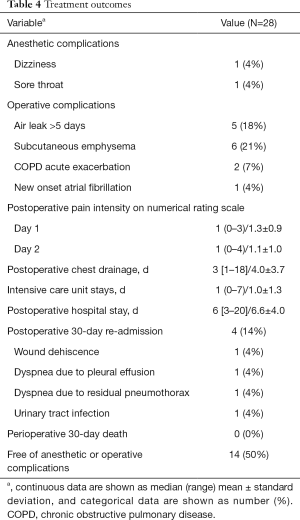
Full table

Full table
Discussion
Our study shows that non-intubated VATS is technically feasible in selected patients with impaired pulmonary function undergoing primary lung cancer surgery. It may be an alternative anesthetic and surgical approach when managing lung cancer patients who are considered high-risk for intubated general anesthesia.
More than half of the resected lung tumors (56%) in our study were primary lung cancers. With the increasing use of low-dose computed tomography screening for early detection of lung cancers, many more patients are emerging as candidates for pulmonary resection for diagnosis or treatment or both (20). Pulmonary resections have historically been avoided in some patients because of pulmonary insufficiency. However, recent studies reveal that marginal pulmonary function should not preclude a surgical approach in selected patients (21,22).
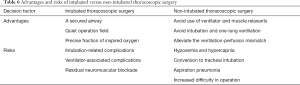
The advantages of non-intubated VATS can be straightforward compared with intubated VATS (13). First, complications related to intubated general anesthesia, such as airway trauma, residual neuromuscular blockade, and ventilator-induced lung injury, can be avoided (10-12,23). Secondly, spontaneous breathing in non-intubated surgery provides favorable respiratory mechanics and alleviates the ventilation-perfusion mismatch during one-lung ventilation (24). Thirdly, avoiding general anesthesia in patients with COPD is associated with lower incidences of morbidity, pneumonia, prolonged ventilator dependence, and unplanned postoperative intubation (25).
Concerns should be considered with the use of non-intubated VATS for patients with impaired pulmonary function (17). First, prolonged spontaneous breathing during non-intubated surgery could lead to hypoxia and hypercapnia. Secondly, conversion to general anesthesia with intubation could be required occasionally. Thirdly, aspiration may occur without protection of the endotracheal tube.
Although hypoxemia may develop during non-intubated VATS, it is mostly transient and can be easily improved with supplemental oxygen via transnasal high-flow humidified oxygen (transnasal humidified rapid-insufflation ventilatory exchange) (26). Hypercapnia was noted in some patients, especially when the surgery was long. Our experience showed that hypercapnia was permissive and did not affect the hemodynamics and surgical procedures (16).
To prevent urgent conversion to tracheal intubation during non-intubated VATS, mainly caused by uncontrollable bleeding, a preplanned protocol of airway management should be kept in mind (17). We suggest that proper patient selection, accumulated experience by performing minor non-intubated thoracoscopic procedures, and conversion to intubated general anesthesia without hesitation are mandatory to decrease the risk of urgent intubation and complications, especially at the beginning of the learning curve. In our experience, patients with vigorous diaphragmatic movement requiring conversion to tracheal intubation were mostly overweight with a BMI >26 kg/m2. We suggest that obese patients are not ideal candidates for non-intubated technique. Without airway protection with tracheal intubation, patients should also be carefully selected to prevent pulmonary aspiration by excluding patients with inadequate fasting, gastroesophageal reflux disease or hiatal hernia. Anticholinergic agents can be given prophylactically to decrease amounts of oral and airway secretions.
In this study, only one 80-year-old male smoker (4%) with COPD required conversion to tracheal intubation because of persistent wheezing and labored breathing. Age was not a determinant factor for non-intubated VATS, but rather airway hyperactivity and vigorous breathing pattern that jeopardizing the surgical field were in this case (27). Smoking is the primary cause of COPD, which comprised 85.7% (24/28) of our study cohort. Airway hyperactivity of COPD and asthma can present with acute exacerbations and result in life-threatening complications peri-operatively (28). Airway instrumentation may lead to bronchoconstriction and is best avoided in high-risk patients (29). In selected patients, the non-intubated technique may allow them to avoid tracheal intubation and further airway irritation. For patients with airway hyperactivity despite maximized pretreatment, however, endotracheal intubation may be unavoidable to deliver inhaled bronchodilators, volatile anesthetics, or both.
Five patients (18%) developed prolonged air leakage requiring lengthy pleural drainage or chemical pleurodesis. All of them had primary lung cancer and emphysema with low FEV1 (Table 5). Prolonged air leakage is the most prevalent postoperative complication with a reported occurrence up to 45.2% in patients with emphysema (30). Non-intubated technique has the theoretical advantage of preserving natural negative-pressure breathing physiology. Additionally, avoiding endotracheal intubation reduces stress to the fragile pulmonary parenchyma frequently observed in patients with impaired lung function. A similar finding was reported by a randomized trial from Pompeo et al., where non-intubated VATS reduces the rate of prolonged air leakage, compared with an intubated group (22% vs. 48%, P=0.036) (14).
We defined patients with impaired lung function as their preoperative FEV1 <70% of the predicted, which is compliant with the moderate impairment of pulmonary function or worse according to the American Thoracic Society guideline (15). There are numerous arbitrary cuff-offs to define lung function impairment in different clinical settings (31,32). A conclusive threshold that has a negative impact on postoperative outcomes is still debated. However, severity of lung function impairment is associated with morbidity and patients with lower FEV1 have more respiratory complaints (33). Their risk of postoperative morbidity and mortality were expected as most of our included patients were categorized as American Society of Anesthesiologists physical status class III or IV (83%).
Our study was limited by its retrospective design with a small number of patients and the lack of a control group for comparison. The short-term safety, efficacy and long-term oncological effects of this technique compared with intubated general anesthetic VATS remains unclear. However, the low intubation conversion rate and acceptable complication rate indicate that non-intubated pulmonary resections can be performed in selected patients with impaired lung function. Further studies should clarify the safety and benefits of this non-intubated technique, or the risk of suboptimal resection and recurrence in lung cancer patients.
In summary, our experience showed that non-intubated VATS is technically feasible. It may be applied as an alternative to intubated general anesthesia in managing lung tumors in selected patients with impaired lung function, after judicious evaluation of the advantages and risks of this procedure (Table 6).
Full table
Acknowledgements
Funding: This work was supported in part by research grants from National Taiwan University Hospital (NTUH104-P08 to Dr. Chen) and the Taiwan Lung Foundation (TLF2015-C02 to Dr. Chen), Taipei, Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Research Ethics Committee of National Taiwan University Hospital (approval No.: 201505004RINA) and written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Wang BY, Huang JY, Cheng CY, et al. Lung cancer and prognosis in taiwan: a population-based cancer registry. J Thorac Oncol 2013;8:1128-35. [Crossref] [PubMed]
- Marcus MW, Raji OY, Field JK. Lung cancer screening: identifying the high risk cohort. J Thorac Dis 2015;7:S156-62. [PubMed]
- Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: A Society of Thoracic Surgeons general thoracic surgery database risk-adjustment model. Ann Thorac Surg 2008;85:1857-65. [Crossref] [PubMed]
- Choi H, Mazzone P. Preoperative evaluation of the patient with lung cancer being considered for lung resection. Curr Opin Anaesthesiol 2015;28:18-25. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: A Society of Thoracic Surgeons database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: A propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013;117:133-41. [Crossref] [PubMed]
- Della Rocca G, Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol 2013;26:40-6. [Crossref] [PubMed]
- Fitzmaurice BG, Brodsky JB. Airway rupture from double-lumen tubes. J Cardiothorac Vasc Anesth 1999;13:322-9. [Crossref] [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis 2014;6:2-9. [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Marshall HM, Bowman RV, Yang IA, et al. Screening for lung cancer with low-dose computed tomography: a review of current status. J Thorac Dis 2013;5 Suppl 5:S524-39. [PubMed]
- Taylor MD, LaPar DJ, Isbell JM, et al. Marginal pulmonary function should not preclude lobectomy in selected patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:738-44. [Crossref] [PubMed]
- Kachare S, Dexter EU, Nwogu C, et al. Perioperative outcomes of thoracoscopic anatomic resections in patients with limited pulmonary reserve. J Thorac Cardiovasc Surg 2011;141:459-62. [Crossref] [PubMed]
- Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: Prospective propensity score matched cohort study. BMJ 2012;345:e6329. [Crossref] [PubMed]
- Yang JT, Hung MH, Chen JS, et al. Anesthetic consideration for nonintubated VATS. J Thorac Dis 2014;6:10-3. [PubMed]
- Hausman MS Jr, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: Does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg 2015;120:1405-12. [Crossref] [PubMed]
- Wang ML, Hung MH, Chen JS, et al. Nasal high-flow oxygen therapy improves arterial oxygenation during one-lung ventilation in non-intubated thoracoscopic surgery. Eur J Cardiothorac Surg 2018;53:1001-6. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Warner DO, Warner MA, Offord KP, et al. Airway obstruction and perioperative complications in smokers undergoing abdominal surgery. Anesthesiology 1999;90:372-9. [Crossref] [PubMed]
- Kim ES, Bishop MJ. Endotracheal intubation, but not laryngeal mask airway insertion, produces reversible bronchoconstriction. Anesthesiology 1999;90:391-4. [Crossref] [PubMed]
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25. [Crossref] [PubMed]
- Osuka S, Hashimoto N, Sakamoto K, et al. Risk stratification by the lower limit of normal of FEV1/FVC for postoperative outcomes in patients with COPD undergoing thoracic surgery. Respir Investig 2015;53:117-23. [Crossref] [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91. [Crossref] [PubMed]
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:14-20. [Crossref] [PubMed]


