
Single-stage hybrid localization: a combination of bronchoscopic lung mapping followed by post-mapping computed tomographic reconstruction and additional transthoracic needle localization in a cone beam computed tomography room
Introduction
Small pulmonary nodules are largely detected due to low-dose computed tomography (CT) screening for lung cancer. For suspicious malignant screened nodules, surgical resection remains the preferred treatment option for both diagnostic and curative intention (1). To identify non-palpable nodules under thoracoscopy, various preoperative localization methods have been used (2). Bronchoscopic lung marking was performed using dye or radiopaque material to localize the target lesion through endobronchial route, which was usually guided under fluoroscopy or conventional multi-detector CT (MDCT) (3,4). The technique of bronchoscopic dye marking has been developed into a specialized approach termed virtual-assisted lung mapping (VAL-MAP), which consisted of virtual bronchoscopic guidance and multiple dye markings for different surgical intentions including wedge resection and segmentectomy (5,6). In VAL-MAP system, fluoroscopic guidance is necessary during bronchoscopic delivery of dye injection catheter to the target pleura, and post-mapping CT scan is recommended for the confirmation of adequate dye injection and three-dimensional (3D) image reconstruction for surgical planning (7).
In recent years, cone beam CT (CBCT)-guided interventions have been used more frequently due to improving image quality of flat panel detector. The CBCT interventional suite can provide real-time fluoroscopic two-dimensional (2D) visualization during bronchoscopic lung mapping, and it also provides 3D images for post-mapping CT reconstruction in the same room. Furthermore, CBCT enables better angle flexibility than MDCT for needle procedures (8), and additional transthoracic needle localization can be performed following post-mapping CT if it is needed. Our institution initiated a VAL-MAP program since May 2017, and we recently modified our work-flow into the single space (9). This study aimed to present our initial experience of bronchoscopic lung mapping followed by post-mapping CT and additional needle localization in a CBCT room.
Methods
Study design and patients
The institutional review board of National Taiwan University Hospital, Hsin-Chu Branch, approved the retrospective retrieval of data for this study (approval number 107-081-E). The study included consecutive patients who underwent preoperative bronchoscopic lung mapping and post-mapping CT with and without additional needle localization procedure in a CBCT room followed by thoracoscopic surgery at our institution between February 2018 and August 2018. At our institute, bronchoscopic lung mapping was indicated for resections of small pulmonary nodules expected to be difficult to identify intraoperatively and for pulmonary segmentectomy.
Workflow
Cone beam CT room setting
The fluoroscopic examination room is equipped with a C-arm CBCT (Artis zeego biplane, Siemens Healthcare GmbH, Forchheim, Germany), which can provide real-time 2D fluoroscopy and 3D CT image through a 6-sec scan protocol with 0.36 µGy/projection and 397 frames acquired over 200° (syngo DynaCT). The C-arm CBCT system is able to adapt to almost any position of the integrated free-floating angiography table, and the C-arm CBCT system can be controlled on the tableside or outside the examination room.
Lung mapping
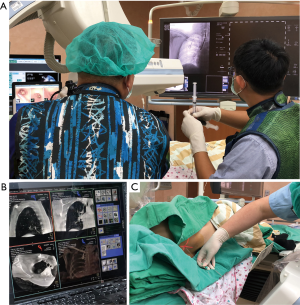
The patients were placed in supine position under local anesthesia and mild sedation. The technique of bronchoscopic dye injection had been described in previous reports (10,11). When the bronchoscope has identified the predetermined target bronchus, the metal-tip catheter is inserted through the working channel of the bronchoscope. The tip of the catheter is confirmed to have reached the visceral pleura using C-arm fluoroscopy with flat panel detector (Figure 1A). After completing the bronchoscopic procedure, a 6-s syngo DynaCT scan is then performed immediately to confirm the location of markings, and the post-mapping CT image is reconstructed into 3D images before surgery.
Needle localization
After lung mapping and post-mapping CT, the additional transthoracic needle procedures can be selectively performed for different purpose, such as direct dye localization to the target lesion and microcoil placement for the deep resection margin. Using syngo Needle Guidance of a syngo X-Workplace (Siemens Healthcare GmbH, Forchheim, Germany) (Figure 1B), the planned needle path was projected with a laser beam on the skin of the patient, and the needle procedure can be performed under laser-supported, fluoroscopic guidance (Figure 1C).
Post-mapping image reconstruction
The post-mapping CBCT images were reconstructed into 3D images including the target lesion and the markings, which usually presented with ground-glass opacities (GGOs) at the anticipated injection sites (Figure 2). The surgical planning was made on the reconstructed 3D image (Figure 3A).
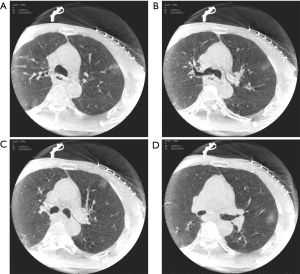
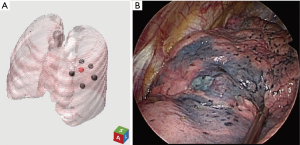
Image-guided thoracoscopic surgery
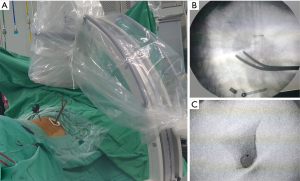
Under direct thoracoscopic visualization, the dye markings could be easily identified (Figure 3B), and the area of resection could be determined according to the location of the dye markings. Additionally, intraoperative fluoroscopy was applied (Figure 4A) to visualize the microcoil and guide the retrieval of adequate resection margin (Figure 4B), and presence of the microcoil in the resected specimen was confirmed via fluoroscopy (Figure 4C).
Data collection and analysis
Preoperative chest CT scans were reviewed, and all lesions were assessed for lobar location, maximum diameter, depth (distance from the nearest visceral pleural surface to the center of the lesion), and appearance on CT [ground-glass nodule (GGN) or solid nodule]. Patient demographics and clinical characteristics were collected from the medical records. Localization-related variables including duration of localization, time from localization to surgery, total number of DynaCT scans taken, duration of fluoroscopy, and total skin dose of radiation exposure were recorded. Postoperative length of stay and pathology were also collected. Descriptive statistics are summarized as medians [interquartile ranges (IQRs)] for continuous data and counts (percentages) for categorical variables.
Results
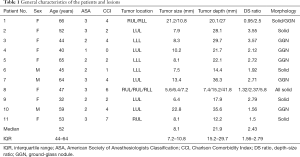
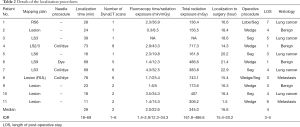
Table 1 shows the general characteristics of the 14 pulmonary lesions in 11 patients included in the case series. According to the preoperative CT findings, 8 (66%) and 6 (33%) lesions were classified as solid lesions and GGNs, respectively. The median size of pulmonary lesions on preoperative CT images was 8.1 mm (IQR, 7.2–10.8 mm), whereas their median distance from the pleural surface was 21.9 mm (IQR, 15.2–29.7 mm). The median tumor depth-to-size ratio was 2.43 (IQR, 1.56–2.79). Table 2 summarizes the details of the lesion localization procedure. The marking procedures were conducted following the mapping plans, which included 5 lesion-directed dye marking and 6 pulmonary segment-directed dye marking. Four additional transthoracic needle localizations were performed after lung mapping, including 3 microcoil placement with superficial dye marking and one dye marking only. The median fluoroscopy duration was 2 min (IQR, 1.4–2.9 min), and the median total localization time was 28 min (IQR, 18–69 min). The median radiation exposure (expressed by the skin absorbed dose) was 345.0 mGy (IQR, 161.8–486.6 mGy). The final pathological diagnoses were as follows: primary lung cancer (n=6), lung metastases (n=4), and benign lung lesions (n=4). The latter group included granulomatous inflammation (n=1), hamartoma (n=1), chronic inflammation (n=1), and smooth muscle metaplasia (n=1). For the 6 primary lung cancer lesions, one lobectomy and 5 segmentectomies were performed. All benign and metastatic lesions were treated with wedge resection. All patients were successfully discharged from the hospital (median length of stay, 4 days; IQR, 3–5 days).
Full table
Full table
Discussion
The bronchoscopic lung mapping system, developed by Sato et al., has been widely used in Japan for various procedures of pulmonary resection (10). Through the combination of virtual bronchoscopy and the simulation of involved bronchial territory, the “mapping mode” of the image software can guide the surgeons to make dye markings on the multiple target bronchi, which provide a lung map for the planned resection, including segmentectomy and wedge resection (6,12). However, the study of CT confirmation after lung mapping had demonstrated that the predicted markings by virtual bronchoscopy were dislocated from the actual markings by an average of 3 cm, and the two different versions of the software showed the same result of discrepancy (7). Therefore, post-mapping CT played an indispensable role to conduct a successful surgery that is guided by lung mapping.
Reviewing current reports of lung mapping, most post-mapping CT were performed by conventional MDCT with thin-slice images (1.00–1.25-mm thickness) within 2 h after mapping procedure. The dye markings usually presented with GGOs with or without bronchial dilation. Compared to MDCT, the noise with lower image quality of CBCT raised some concerns in clinical use (13), especially when identifying some obscure findings such as GGOs. In our initial experience, determining the actual marking locations on post-mapping CBCT images with lung window settings (window level: −500 HU, window width: 1,500 HU) was clinically straightforward, as long as the typical CT appearance of dye marking was known. Accordingly, the determined locations of each marking was plotted on the pre-mapping CT images based on the structural characteristics of pulmonary vessels and bronchioles, and the images were reconstructed into 3D images with the lesion and markings included.
In our series, the radiation exposure increased mainly because of the number of DynaCT. For lung mapping only, DynaCT could be performed only once as post-mapping CT; otherwise, in some cases, DynaCT was performed additionally to confirm the presence of the lesion before the mapping procedure. For those receiving additional needle procedures, DynaCT up to 8 times was performed for both dye injection and microcoil placement. Even though syngo Needle Guidance system was applied to our practice, the number of DynaCT did not decrease during needle procedure, which could be attributed to the preference of our radiologist, who usually performs needle localization under guidance of conventional MDCT with repeated CT scans for confirming the location of the needle. In recent studies, the number of DynaCT could be limited to 2–3 for a single dye or hookwire localization by utilizing real-time fluoroscopy to guide needle passage and reaching to the target (14).
Our previous report (9) had demonstrated a prototype of multimodality image-guided thoracic surgery, which could be transferred to the hybrid operating room (HOR). By using C-arm CBCT, the bronchoscopic and transthoracic needle procedures can be combined into single step like our series; however, still many CBCT rooms that were originally build for angiography procedures are not qualified for surgery. Moreover, time is needed for the confirmation of localization results, and the reconstruction of 3D images, which is essential for successful surgical planning, and efficient image-processing tools, such as specialized work station, have the key role to finalize the protocol of the single-step surgery in HOR.
This study has several limitations. First, this is a single-centered, retrospective designed study with a small sample size. Second, the lack of a control group (performing bronchoscopic and CT-guided procedure separately) makes it difficult to differentiate the specific benefits of combining the workflows into one step. Third, the procedures were not well standardized in some parts, especially when performing needle localization, and more cases are necessary to overcome the learning curve of using the iGUIDE system (15). Further investigations are necessary to clarify the applicability and benefits of this new localization method.
In conclusion, bronchoscopic lung mapping followed by post-mapping CT and additional needle localization can be performed altogether in a single examination room equipped with C-arm CBCT, which provides all the necessities of image guidance, including real-time fluoroscopy and CT scan, and the results of localization are contributory to the surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of National Taiwan University Hospital, Hsin-Chu Branch, approved the retrospective retrieval of data for this study (approval number 107-081-E).
References
- Yang SM, Hsu HH, Chen JS. Recent advances in surgical management of early lung cancer. J Formos Med Assoc 2017;116:917-23. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Sakamoto T, Takada Y, Endoh M, et al. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg 2001;72:296-7. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography-guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Sato M, Nagayama K, Kuwano H, et al. Role of post-mapping computed tomography in virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2017;25:123-30. [Crossref] [PubMed]
- Yang SM, Ko WC, Lin MW, et al. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J Thorac Dis 2016;8:S681-9. [Crossref] [PubMed]
- Yang SM, Lin CK, Chen LW, et al. Combined virtual-assisted lung mapping (VAL-MAP) with CT-guided localization in thoracoscopic pulmonary segmentectomy. Asian J Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Sato M. Virtual assisted lung mapping: navigational thoracoscopic lung resection. Cancer Res Front 2016;2:85-104. [Crossref]
- Sato M, Yamada T, Menju T, et al. Virtual-assisted lung mapping: outcome of 100 consecutive cases in a single institute. Eur J Cardiothorac Surg 2015;47:e131-9. [Crossref] [PubMed]
- Sato M, Aoyama A, Yamada T, et al. Thoracoscopic wedge lung resection using virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2015;23:46-54. [Crossref] [PubMed]
- Fave X, MacKin D, Yang J, et al. Can radiomics features be reproducibly measured from CBCT images for patients with non-small cell lung cancer? Med Phys 2015;42:6784-97. [Crossref] [PubMed]
- Hsieh MJ, Fang HY, Lin CC, et al. Single-stage localization and removal of small lung nodules through image-guided video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2017;53:353-8. [Crossref] [PubMed]
- Hsieh MJ, Wen CT, Fang HY, et al. Learning curve of image-guided video-assisted thoracoscopic surgery for small pulmonary nodules: a prospective analysis of 30 initial patients. J Thorac Cardiovasc Surg 2018;155:1825-32.e1. [Crossref] [PubMed]


