Understanding the link between traumatic brain injury and Alzheimer’s disease
Due to the multitude of failed clinical trials for neurodegenerative diseases, many prominent researchers have begun to re-examine what we know about these diseases and how they progress. A recent review article by Ramos-Cejudo and colleagues discussed the contribution of cerebrovascular dysfunction following traumatic brain injury as a potential mechanism leading towards neurodegeneration (1). While the article brought up several important points that will be discussed below, it was limited in the full characterization of injury expansion and failed to mention some key markers that warrant further investigation. In this editorial, we will highlight the compelling evidence for cerebrovascular dysfunction as a contributor to neurotrauma related neurodegeneration, while emphasizing some other important molecular pathways to consider that are emerging as crucial players for injury progression.
It is well known that amyloid beta and tauopathy are key markers of neurodegeneration (2). What is unknown is how the cascade is started in order to progress towards neurodegeneration. Some groups have investigated the genetic link (3), while others have looked at more inciting events (stress, neural injury, drug abuse, etc.). More and more evidences are linking traumatic brain injury to the development of later onset neurodegeneration. Differentiating Alzheimer’s disease from chronic traumatic encephalopathy has been a challenge and has relied primarily on post-mortem pathology (4). As Ramos-Cejudo aptly pointed out, cerebrovascular dysfunction is often the initial injury mechanism that gets the ball rolling towards neurodegeneration. The injury is often subtle in the form of acute blood brain barrier disruption (5). This blood brain barrier disruption initiates a host of downstream processes.
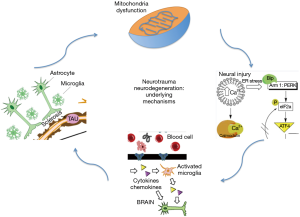
One of the most important initial injury markers related to blood brain barrier disruption is astrogliosis. Astrocytes surround the brain vasculature and become activated once the vessel is injured from shear stress. Tight junction proteins are disrupted allowing inflammatory markers from the blood stream to interact with astrocyte podocytes. The activated astrocyte becomes gliotic once a certain threshold of neuroinflammation is reached (6). How this process unfolds is not completely understood, but emerging evidence has linked pericytes as key regulators for accelerating gliosis. While astrogliosis has long been reported following traumatic brain injury, little was known about the underlying mechanisms that occur due to astrogliosis at the cellular level. One of the earliest responses associated with astrogliosis is the activation of oxidative stress. Oxidative stress produces damage to both cellular membranes as well as internal organelles such as the endoplasmic reticulum and mitochondria. Acutely this has two primary effects. First, the damaged endoplasmic reticulum leads to the unfolded protein response leaving the cell stuck in a stunned state. The unfolded proteins are prone to biochemical modifications such as phosphorylation, acetylation, and nitration. Second, these damaged cells release a host of inflammatory cytokines into the brain milieu that activate nearby microglia (7). The recruited microglia cross talk with the gliotic astrocytes. Overtime protein aggregates from the gliotic cells are excreted into the surrounding milieu actively or from dying cells accelerating the process of neuroinflammation.
The persistent neuroinflammation process starts off the tau progression pathway. Damaged axons from the initial shear stress of the trauma begin to form paired helical filaments based on cytokine signaling from the surrounding inflammatory milieu (8). The released protein aggregates and oxidative stress from the gliotic blood brain barrier cells damage surrounding neuronal membranes. The damaged membranes are then susceptible to the uptake of aggregates into the cell. This process of protein aggregate uptake into neurons is primarily orchestrated by microglia phenotype switching from the M1 to M2 subtype. The stunned neuron then activates a host of kinases. Once the initial tau paired helical filaments form within the axon, the tau particles migrate into the soma and begin to formulate oligomers. The increased kinases within the soma hyperphosphorylate these oligomers making them susceptible to further biochemical modification (9). One of the key components that mark the change towards neurodegeneration is tau acetylation. Acetylated tau oligomers are irreversibly modified, which contributes to the development of neurofibrillary tangles.
After the tauopathy cascade is initiated other protein aggregates such as amyloid Beta and TDP-43 accumulate within the damaged neurons. In a healthy brain, many of these aggregates are excreted into the surrounding milieu and are flushed out of the parenchyma through the glymphatic system (10). If too much damage has occurred, the glymphatic system become clogged and the aggregates begin to accumulate within cells and the surrounding milieu, which leads surrounding cells to begin the degenerative process as well. Age accelerates the progression of this process through cerebrovascular disease, atherosclerosis, and further disruption of glymphatic clearance mechanisms. The initial pathology is evident most prominently where the injury occurred, but quickly spreads along known axonal connection pathways.
Two mechanisms make initially healthy neurons susceptible to damage from nearby injured cells. The first is endoplasmic reticulum stress. Typically, the endoplasmic reticulum plays an important role in protein folding and cellular homeostasis. If the internal calcium signaling is disrupted from persistent neuroinflammation and oxidative stress, the endoplasmic reticulum is no longer able to fold proteins (11). Once this occurs, the cell becomes stunned making it susceptible to the degenerative changes seen in tauopathies, such as Alzheimer’s disease. The second pathway that occurs simultaneous as Ramos-Cejudo point out is mitochondrial dysfunction. Mitochondria can also become damaged from oxidative stress and neuroinflammation (12). Once this occurs, the cell loses its ability to successfully function. The neuron’s workhorse no longer provides enough energy for active clearance of aggregated proteins. This leads to aggregate build up within the cell until it eventually dies.
Going forward it will be critical to target the underlying mechanisms regulating neurotrauma related neurodegeneration early before the disease manifests clinically (Figure 1). Initially following injury, oxidative stress and endoplasmic reticulum stress should be targeted pharmacologically in order to mitigate the injury expansion. Once the initial signs of tauopathy become apparent, the disease is likely incurable. The progression can be slowed however by targeting neuroinflammation (13). Reducing the inflammatory milieu within the brain will hinder progression of cell damage along the axonal tracts. Finally, restoring both intracerebral vascular function as well as glymphatic clearance should be at the forefront of ongoing research.
Advances in endovascular therapies have already provided groundbreaking treatment options for ischemic stroke, aneurysms, and decreasing vascular flow to tumors. As the pharmaceutical options improve in coming years, endovascular targeted therapy will provide focused treatment for restoring tight junction protein adherence and preventing astrogliosis immediately following traumatic brain injury. Similar to verapamil being injected adjacent to an artery in vasospasm, we will be able to release drugs adjacent to sites of injury following traumatic brain injury. One promising pathway to target would be caveolin signaling. Caveolin upregulation has been shown to increase peripheral immune cell diapedesis across damaged blood brain barrier locations (14). This not only accelerates neuroinflammation but also intensifies the astrogliosis. Preventing this mechanism pharmaceutically warrants further investigation.
After the injury has already occurred and aggregates begin to develop, focused intracranial ultrasound can be utilized to enhance glymphatic clearance (15). The focused ultrasound will help clear the sludge buildup and facilitate restoration of the brains ability to excrete toxic protein aggregates into the blood stream for clearance. With advances in targeted ultrasound, the treatment can be focused to sites known injury sites or sites that are common end-pathways for Alzheimer’s disease. In addition, by facilitating clearance into the blood stream, clinicians will be able to better detect specific protein aggregates improving treatment approaches.
One emerging area related to early detection of injury is biomarkers. Recent evidence has emerged that microRNA are upregulated following cerebrovascular injury. Detecting these microRNA changes early following injury will be critical for determining what patients can most benefit from treatment. Specific microRNAs of interest are part of the let7 family (16). This family of microRNAs can regulate important pathways related to blood brain barrier disruption, oxidative stress, and neuroinflammation, the importance of which has been highlighted above. Therefore, using these markers to guide treatment will serve as an excellent avenue for transitioning to the new area of Alzheimer’s management. Previous clinical trials have all targeted end products in the disease process such as amyloid beta and tau. Targeting the early molecular pathways as highlighted above is a much better approach in that disease modification can truly take place.
In summary, cerebrovascular dysfunction plays an important role in neurotrauma related neurodegeneration. While amyloid beta and tau are important markers, earlier biochemical changes mediate the disease process. Targeting these early biochemical changes and preventing the subsequent protein aggregation will be critical in establishing effective treatments. The available treatments will be both pharmaceutical and procedural based in order to provide best efficacy. Going forward future research is warranted in order to fully establish the biochemical pathways contributing to neurotrauma related neurodegeneration.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ramos-Cejudo J, Wisniewski T, Marmar C, et al. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018;28:21-30. [Crossref] [PubMed]
- Woerman AL, Aoyagi A, Patel S, et al. Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci U S A 2016;113:E8187-96. [Crossref] [PubMed]
- Sayed FA, Telpoukhovskaia M, Kodama L, et al. Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proc Natl Acad Sci U S A 2018;115:10172-7. [Crossref] [PubMed]
- Turner RC, Lucke-Wold BP, Robson MJ, et al. Alzheimer’s disease and chronic traumatic encephalopathy: Distinct but possibly overlapping disease entities. Brain Inj 2016;30:1279-92. [Crossref] [PubMed]
- Logsdon AF, Lucke-Wold BP, Turner RC, et al. A mouse Model of Focal Vascular Injury Induces Astrocyte Reactivity, Tau Oligomers, and Aberrant Behavior. Arch Neurosci 2017.4. [PubMed]
- Lananna BV, Nadarajah CJ, Izumo M, et al. Cell-Autonomous Regulation of Astrocyte Activation by the Circadian Clock Protein BMAL1. Cell Rep 2018;25:1-9.e5. [Crossref] [PubMed]
- Abe N, Choudhury ME, Watanabe M, et al. Comparison of the detrimental features of microglia and infiltrated macrophages in traumatic brain injury: A study using a hypnotic bromovalerylurea. Glia 2018;66:2158-73. [Crossref] [PubMed]
- Bodnar CN, Morganti JM, Bachstetter AD. Depression following a traumatic brain injury: uncovering cytokine dysregulation as a pathogenic mechanism. Neural Regen Res 2018;13:1693-704. [Crossref] [PubMed]
- Holzer M, Schade N, Opitz A, et al. Novel Protein Kinase Inhibitors Related to Tau Pathology Modulate Tau Protein-Self Interaction Using a Luciferase Complementation Assay. Molecules 2018.23. [PubMed]
- Opel RA, Christy A, Boespflug EL, et al. Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J Cereb Blood Flow Metab 2018:271678X18791632.
- Hylin MJ, Holden RC, Smith AC, et al. Juvenile Traumatic Brain Injury Results in Cognitive Deficits Associated with Impaired Endoplasmic Reticulum Stress and Early Tauopathy. Dev Neurosci 2018;40:175-88. [Crossref] [PubMed]
- Saha P, Gupta R, Sen T, et al. Activation of cyclin D1 affects mitochondrial mass following traumatic brain injury. Neurobiol Dis 2018;118:108-16. [Crossref] [PubMed]
- Schimmel SJ, Acosta S, Lozano D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ 2017;3:135-42. [Crossref] [PubMed]
- Wang X, Ren X, Wang Y, et al. Traumatic brain injury research and expression of caveolin-1 and its relationship with disease prognosis. Pak J Pharm Sci 2017;30:997-1000. [PubMed]
- Shen WB, Anastasiadis P, Nguyen B, et al. Magnetic Enhancement of Stem Cell-Targeted Delivery into the Brain Following MR-Guided Focused Ultrasound for Opening the Blood-Brain Barrier. Cell Transplant 2017;26:1235-46. [Crossref] [PubMed]
- Toffolo K, Osei J, Kelly W, et al. Circulating microRNAs as biomarkers in traumatic brain injury. Neuropharmacology 2018. [Epub ahead of print]. [Crossref] [PubMed]