The effectiveness of allogeneic mesenchymal stem cells therapy for knee osteoarthritis in pigs
Introduction
Knee osteoarthritis (OA) is a common chronic disease and a major cause of disability in people aged 45 and above (1). Management of knee OA can be roughly divided into nonsurgical and surgical interventions. The choice of treatment is based on symptoms, OA stage, and patient-related factors (2). Referral for consideration of surgical intervention (osteotomy, arthroscopic interventions, and knee arthroplasty) is indicated after more conservative treatment options have been exhausted (3). Surgical treatments such as total knee arthroplasty, are more suitable for patients with late stage knee OA; however this procedure may lead to the joint disfunction (4). Nonsurgical treatments include lifestyle modifications and exercise (losing weight, switching from running or jumping exercises to swimming or cycling), pharmacologic therapies (complementary and alternative medicine, nonsteroidal anti-inflammatory drugs), and physiotherapy (kneading and hot compress) (2,5).
Cell therapy and tissue engineering approaches have become an alternative option for treating cartilage defects (4). Intra-articular injections may include administration of stem cells collected from different sources, platelet-rich plasma (PRP), hyaluronan preparations, and ozone (6,7). Mesenchymal stem cells (MSC) are superior to others due to: (I) the self-renewal ability; (II) being essential for normal turnover and maintenance of cartilage; (III) being capable to migrate to the damaged area of cartilage; and (IV) having the ability to induce chondrocyte proliferation and extracellular matrix (ECM) synthesis (4).
There are a plethora of reports on management of knee OA using MSC which have reported conflicting results mainly due to heterogeneous methodologies used (8). In several studies bone marrow MSCs (BM-MSCs) has been used as a source of MSCs. Thus, an effective in vivo cell tracer technology is necessary to distinguish the donor MSCs and to observe its migratory pathway to the receptors. At present, there are two methods that can be applied for tracing the cell, namely nuclear medicine and magnetic resonance imaging (MRI) (9). Nuclear medicine imaging technology is characterized by high sensitivity and specificity, but has the disadvantages of radioactive decay, short imaging time and low spatial resolution; while MRI can provide a resolution of up to 25–50 µm (9). Therefore, MRI has the high spatial and temporal resolution, and promising application prospect for real-time cell tracing. For tracing transplanted cells in vivo with MRI, labeling transplanted cells with contrast medium and high safety is an important issue (10). Currently, the contrast agents used for stem cell magnetic labeling are mainly based on magnetic iron oxide as the negative contrast agent, resulting in a strong T2 negative contrast effect (11). Contrast agents are divided into two types according to the size of the particle diameter: superparamagnetic iron oxide (SPIO) with diameter greater than 50 nm, and ultra-small superparamagnetic iron oxide (USPIO) with a diameter that is less than 50 nm (12). SPIO has been widely used for labeling transplanted cells due to its low threshold concentration and small cytotoxicity to MRI (13).
The aim of the present study was to label SPIO to allogenic BM-MSCs in order to examine the cell migration and therapeutic effect in pig OA model.
Methods
Study design
Six female bama miniature pigs, 150–180 days old, weighing 20–25 g, were obtained from Pudong New Area, Shanghai, China [SCXK(HU)2013-0013] [SYXK(SU)2014-0051]. All animal studies (including the mice euthanasia procedure) were done in compliance with the regulations and guidelines of Nanjing University institutional animal care and were conducted according to the AAALAC and the IACUC guidelines [SYXK(SU)2014-0051].
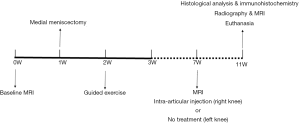
MRI was used to examine the knee joints in all pigs. One week later, bilaterally medial meniscectomy and guided exercise (which lasted 7 days) were used to induce knee OA, after which pigs were allowed to move freely. Seven weeks later, the labeled BM-MSCs were injected into the right knee, while the left side was left untreated. The MRIs were carried out once a week for the next 28 days, after which all animals were euthanized, and knee samples were collected and further examined. The study designs are shown in Figure 1.
Preparation of SPIO labeled BM-MSCs
Allogenic porcine BM-MSCs (CELLBIO, CBR-131561) were used for intra-articular injection. Certain concentration of SPIO nanoparticles were used to label the cells for 24 h, to ensure real-time cells tracking using the MRI.
Cell viability
BM-MSCs were seeded in a 96-well plate at the density of 1×104 cells per well and cultured for 12 h for adherence. Cells were then exposed to gradually increased concentration (0, 2.5, 5, 10, 20 and 40 µg/mL) of SPIO for 24 h, after which they were examined using a Cell Counting Kit-8 (CCK-8) (KeyGen BioTech, Nanjing, China) according to the manufacturer’s protocol.
Examining intracellular SPIO
For confirming the intracellular SPIO, the BM-MSCs were seeded on gelatin coated slides in 24-well plate at the density of 1×105 cells per well and cultured for 12 h for adherence. Next, the cells were exposed to gradually increased concentration (0 and 20 µg/mL) of SPIO for 72 h. The slides were then washed with PBS and stained with Prussian blue staining (Solarbio, Beijing, China), according to the standard protocol. For in vivo experiment, the BM-MSCs were labeled with SPIO at the concentration of 20 µg/mL and cultured for 72 h.
Intra-articular injection of allogenic BM-MSCs
A total of 1×107 BM-MSCs (3 mL) were injected into the right medial compartment of the right knee joint once a week for a duration of 4 weeks. The joint was repeatedly flexed and extended to ensure cell dispersions throughout the joint after every injection.
MRI & radiography
Pre-detections were carried out before each surgery. Pigs were first anesthetized with 3.5 mg/kg Xylazine and the MRI (uMR770 3.0T, United Imaging, Shanghai, China) to evaluate the articular cartilage. Eight, 24 and 120 h post-injection, the knee joints were separately assessed using MRI. At the 11th week, the pigs were sacrificed and the isolated knee joints were appraised according to the Kellgren and Lawrence (K/L) system and Osteoarthritis Research Society International (OARSI) atlas (14).
Macroscopic examination, histological analysis & immunohistochemistry
All pigs were euthanized, and their knee joints were isolated and evaluated according to the International Cartilage Repair Society (ICRS) macroscopic score (15,16). Then, the samples were fixed in 4% paraformaldehyde for 24 h and decalcified in 10% methanoic acid for 1 month. Next, the tissues were embedded and sectioned into 5 µm thick slices. After that, the slices were stained with hematoxylin and eosin (H&E), safranin-o fast green staining and toluidine blue, and were evaluated according to the OARSI Osteoarthr Cartil Histopathology Assessment scoring system (17,18). Sections were covered with anti-collagen type I, II, and X antibody (Sigma Aldrich, USA) and incubated at 4 °C overnight. The sections were reacted with the secondary antibody (Sigma Aldrich, USA) for 30 min at room temperature. Immunohistochemical staining of type I, II, and X were detected with the Vectastain ABC reagent (Vector Laboratories, USA).
Statistical analysis
Statistical analysis was performed using SPSS v22.0 (SPSS Inc., Chicago, IL, USA) and graphs were done with GraphPad Prism v7.0 (GraphPad Software Inc., La Jolla, CA, USA). Comparisons between the therapy side and the control side of the knee joint were preformed using unpaired t-test. A P value of <0.05 was considered statistically significant.
Results
The effect of SPIO on BM-MSCs viability
The effects of the SPIO on cell viability of bone marrow stem cells (BMSCs) are shown in Figure 2. Compared to concentration of 0–20 µg/mL, decreased cell proliferation was observed at concentration of 40 µg/mL.
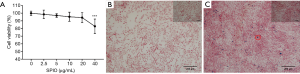
To determine the presence of the particles within the cell, BMSCs were stained with Prussian blue. Briefly, numerous blue granules were found within the cytoplasm after exposure to 20 µg/mL SPIO for 72 h (Figure 2), while no Prussian blue-positive particles were observed in control group.
MRI & radiography
No cartilage damage was found before surgery (Figure 3A). Consequently, after preforming meniscectomy, the medial meniscus was observed (Figure 3B). In addition, the MRI demonstrated typical OA changes of ruffled and thinner local cartilage (red arrow in Figure 3C), as well as subchondral bone cystic degenerations (white arrow in Figure 3C).
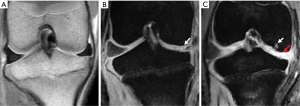
Next, BM-MSCs were injected into the right medial compartment of the right knee joint once a week for a duration of 4 weeks. The movement of cells was examined using MRI. BM-MSCs were found moving towards the impaired part of the cartilage 8 to 24 h after injections. The SPIO labeled BM-MSCs showed special signals on the MRI (Figure 4).
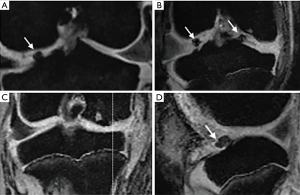
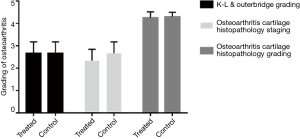
As shown in Figure 5, 4 weeks post-injection, osteophytes and osteosclerosis were still visible around condyles and the tibial plateau. The results of K-L & Outer bridge grading of the knee are shown in Figure 6. In addition, no differences between the two groups were observed.
Macroscopic examination, histological analysis & immunohistochemistry
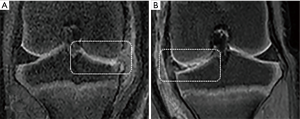
The gross specimens of knee joints were harvested after 4 weeks of injections. Dim rupture was found on the surface of both right and left side of the cartilage, however no significant difference between the right and left side was observed (Figures 6,7).
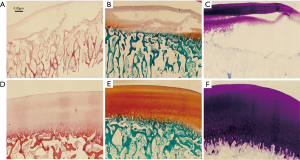
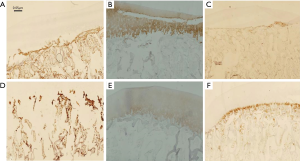
Stained slices revealed the damage of the cartilage on both sides (Figure 7); again, no significant difference was found between the two groups. The immunohistochemical staining of femoral condylar cartilages showed the similar results (Figure 8).
Discussion
In the present study, we preformed meniscectomy to establish a pig OA model, and to evaluate the effectiveness of allogenic MSCs in treating OA. The obtained results revealed that 20 µg/mL of SPIO was a suitable level for labelling BM-MSCs. The SPIO labeled BM-MSCs were found moving towards the impaired part of the cartilage 8 to 24 h after the injections. Yet, there were no significant differences between the treatment and control group.
Animal models are commonly used to study the pathogenesis, treatment and prevention of OA (19). There are many kinds of animal OA models, which are selected according to different study objectives (19). Certain models imply opening the articular capsule and damaging the intraarticular structures, such as cruciate ligament, meniscus, articular cartilage and so on (20), while others can be obtained via intra-articular injection of drugs that trigger OA (21). Although different models induce different mechanisms, ultimately cartilage degenerative changes and secondary bone changes occur (22). Small animals such as mice have a selection advantage when they need to be used in large quantities. Large animal models are suitable for the progression of OA, as their anatomical structure and mechanical structure are similar to the human structures (19-21). It has been demonstrated that pig models are suitable for the study of pathological process, histopathological characteristics, or changes in cartilage biochemical metabolism of OA (23-27). Partial or complete resection of the meniscus is a common method used for establishing an animal model of OA. After meniscal resection, the corresponding load of the patellofemoral joint is disordered, and the compressive stress is concentrated in an extremely small area, resulting in degenerate, rough fibrosis, or complete absence of the articular cartilage (28). Previous studies have shown that guiding the pig to exercise after meniscectomy could increase the load on the cartilage, thus accelerating the wear of the cartilage and inducing OA in a relatively short period of time (20).
Stem cell source, dosage, types of cell transplants (allogenic or autologous), and the feasibility of multiple injections are major issues related to BM-MSCs. The effects of MSC in treating cartilage degeneration and injury have been repeatedly reported in both, animal models and patients. Nevertheless, scholars and doctors have not yet reached a consensus. Jo and colleagues have reported that a high dose (1.0×108 AD MSCs) could improve the knee function in patients with OA (29). A multicenter randomized controlled clinical trial has also revealed that the similar dose of BM-MSCs together with hyaluronic acid lead to clinical and functional improvement (4). In the present study, the effectiveness of BM-MSCs was certainly worth considering. The intra-articular injection group did not show any significant differences in histological changes, which might be due to the subchondral bone damage, which has an import role in OA (30). Changes in subchondral bone can lead to the damage of cartilage. MRI showed collapse and cyst in subchondral bone, which suggested a late-stage of osteoarthritis. Some studies have indicated a therapeutic effect of MSCs in early stage of OA (31). On the other hand, the allogenic BM-MSCs were used, unlike the common autologous ones. Allogenic BM-MSCs are easily manufactured in a large scale and are widely used in clinical applications. Udehiya and colleagues have illustrated a similar result of allogenic and autogenic BM-MSCs in repairing segmental bone defects in rabbits (32). Yang et al. have reported a protective role of allogenic BM-MSCs in the early stage of OA (31). Although we believed that allogenic BM-MSCs had promising application potential for treating OA, the obtained results were not consistent with our expectations. We did not find a significant difference between treatment and control group.
BM-MSCs, which are ideal for bone and cartilage tissue engineering, are more easily accessed, have more rapid expansion, and less immunological rejection (4). Some researchers believe that the MSCs can attach to the surface of cartilage and differentiate into chondrocytes to repair the defects, which is also known as stem cell homing (33). Stem cell homing includes four successive processes: cell recruitment, cell migration, cell proliferation and cell differentiation. Previous studies have shown that many tissues like heart, liver, brain, nerve, kidney, and epithelium go through the homing process of MSCs to promote the repair of injury (16), and have the potential for multi-directional differentiation, with highly self-renewing and proliferative ability (34). Still, the mechanism of stem cell homing remains unclear. In the present study, we found the directional migration of the MSCs in vivo. As Figure 4 shows, BM-MSCs could be found in the articular cavity 8 h after injection. At 24 h after injection, it was possible to see the accumulation of BM-MSCs near the damaged side of the cartilage. Also, there was no obvious special signals till the 5th day after the injection.
The fact that BM-MSCs had no effect or were relatively inefficient could be explained by the following hypotheses: the intracellular concentration of SPIO could be diluted as a result of cell proliferation and differentiation (35); the mobility of joint might damage the BM-MSCs and then affect the biological activity of BM-MSCs. Furthermore, the SPIO released from dead cells might be phagocytosed by macrophages, which could also have an influence on the results of MRI. Arbab and other studies have found that iron is rapidly metabolized or degraded when iron-bearing cells die or macrophages phagocytize iron ions (36). At this time, the decrease of local MRI signal mainly occurs due to release of iron after cell death, suggesting that MRI low signal may be derived from iron containing cells, or from the iron released from dead cells, or from the hemoglobin in the bleeding process. Recently, there has been some debate about whether SPIO affects the chondrogenic differentiation of stem cells (37,38), which is why we selected the acceptable maximum dose (20 µg/mL) in order to guarantee a higher survival rate of cells, and to ensure a clearer MRI image. However, we only tested the ability of these cells to induce differentiation in the culture dish. Whether they have a tissue forming ability and whether they can be used as a tissue engineered seed cell for the repair of tissue defects needs to be further examined.
The present study has some limitations that need to be pointed out. First, the sample size was small, since the animal facility we used did not have enough room to keep too many pigs at the same time. Second, the pigs were not fastened after injection, which might have influenced the viability of BM-MScs. Third, we did not make the comparison of allogenic and autologous cells in one model, thus making the results less convincing. Moreover, we did not take the severity of OA into account, which might have impacted the treatment effects. Moreover, it was impossible to objectively evaluate the subjective feelings of the pigs using scoring scales.
In conclusion, 20 µg/mL of SPIO was the most suitable concentration for labelling BMSCs and the labelled allogenic BM-MSCs could be seen moving towards and accumulating around the lesion area of the cartilage. Yet, allogenic BM-MSCs was not significantly different between the treated group and control group. Thus, more studies are required to further investigate the therapeutic effect of allogeneic BM-MSCs in vivo.
Acknowledgements
Funding: This study was supported by the International Cooperation and Exchanges NSFC (81420108021), Key Program of NSFC (81730067), NSFC (51575100), NSFC (51705259), Excellent Young Scholars NSFC (81622033), Jiangsu Provincial Key Medical Center Foundation and Jiangsu Provincial Medical Outstanding Talent Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All animal studies (including the mice euthanasia procedure) were done in compliance with the regulations and guidelines of Nanjing University institutional animal care and were conducted according to the AAALAC and the IACUC guidelines [SYXK(SU)2014-0051].
References
- Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthr Cartil 2018;26:319-25. [Crossref] [PubMed]
- Schiphof D, van den Driest JJ, Runhaar J. Osteoarthritis year in review 2017: rehabilitation and outcomes. Osteoarthr Cartil 2018;26:326-40. [Crossref] [PubMed]
- Buttgereit F, Burmester GR, Bijlsma JW. Non-surgical management of knee osteoarthritis: where are we now and where do we need to go? RMD Open 2015;1. [Crossref] [PubMed]
- Lamoespinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2016;14:246. [Crossref] [PubMed]
- Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 2004.S6-15. [Crossref] [PubMed]
- Jubert NJ, Rodriguez L, Reverte-Vinaixa MM, et al. Platelet- Rich Plasma Injections for Advanced Knee Osteoarthritis A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop J Sports Med 2017;5. [PubMed]
- Cc LD, Dos Santos FC, Lmob DJ, et al. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. Plos One 2017;12. [Crossref] [PubMed]
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 2014;32:1254. [Crossref] [PubMed]
- Unger EC. How can superparamagnetic iron oxides be used to monitor disease and treatment? Radiology 2003;229:615-6. [Crossref] [PubMed]
- Sun R, Dittrich J, Le-Huu M, et al. Physical and biological characterization of superparamagnetic iron oxide- and ultrasmall superparamagnetic iron oxide-labeled cells: a comparison. Invest Radiol 2005;40:504-13. [Crossref] [PubMed]
- Kirchin MA, Runge VM. Contrast agents for magnetic resonance imaging: safety update. Top Magn Reson Imaging 2003;14:426-35. [Crossref] [PubMed]
- Oude Engberink RD, van der Pol SM, Dopp EA, et al. Comparison of SPIO and USPIO for in vitro labeling of human monocytes: MR detection and cell function. Radiology 2007;243:467-74. [Crossref] [PubMed]
- Sosnovik DE, Schellenberger EA, Nahrendorf M, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med 2005;54:718-24. [Crossref] [PubMed]
- Culvenor AG, Engen CN, Øiestad BE, et al. Defining the presence of radiographic knee osteoarthritis: a comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg Sports Traumatol Arthrosc 2015;23:3532-9. [Crossref] [PubMed]
- Hoemann C, Kandel R, Roberts S, et al. International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials. Cartilage 2011;2:153-72. [Crossref] [PubMed]
- van den Borne MP, Raijmakers NJ, Vanlauwe J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr Cartil 2007;15:1397-402. [Crossref] [PubMed]
- Kraus VB, Huebner JL, Degroot J, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr Cartil 2010;18:S35-52. [Crossref] [PubMed]
- Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthr Cartil histopathology. Osteoarthr Cartil 2006;14:13-29. [Crossref] [PubMed]
- Teeple E, Jay GD, Elsaid KA, et al. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J 2013;15:438-46. [Crossref] [PubMed]
- Cruz R, Ramirez C, Rojas OI, et al. Menisectomized miniature Vietnamese pigs develop articular cartilage pathology resembling osteoarthritis. Pathol Res Pract 2015;211:829-38. [Crossref] [PubMed]
- Whitelaw CB, Sheets TP, Lillico SG, et al. Engineering large animal models of human disease. J Pathol 2016;238:247-56. [Crossref] [PubMed]
- Watson AL, Carlson DF, Largaespada DA, et al. Engineered Swine Models of Cancer. Front Genet 2016;7:78. [Crossref] [PubMed]
- Schlichting N, Dehne T, Mans K, et al. Suitability of Porcine Chondrocyte Micromass Culture To Model Osteoarthritis in Vitro. Mol Pharm 2014;11:2092-105. [PubMed]
- Bichara DA, Pomerantseva I, Zhao X, et al. Successful creation of tissue-engineered autologous auricular cartilage in an immunocompetent large animal model. Tissue Eng Part A 2014;20:303-12. [Crossref] [PubMed]
- Isogai N, Kusuhara H, Ikada Y, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng 2006;12:691-703. [Crossref] [PubMed]
- Lübke C, Ringe J, Krenn V, et al. Growth characterization of neo porcine cartilage pellets and their use in an interactive culture model. Osteoarthr Cartil 2005;13:478-87. [Crossref] [PubMed]
- Dehne T, Schenk R, Perka C, et al. Gene expression profiling of primary human articular chondrocytes in high-density micromasses reveals patterns of recovery, maintenance, re- and dedifferentiation. Gene 2010;462:8. [Crossref] [PubMed]
- Ahern BJ, Parvizi J, Boston R, et al. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartil 2009;17:705-13. [Crossref] [PubMed]
- Jo CH, Chai JW, Jeong EC, et al. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am J Sports Med 2017;45:2774-83. [Crossref] [PubMed]
- Funck-Brentano T, Cohen-Solal M. Subchondral bone and osteoarthritis. Curr Opin Rheumatol 2015;27:420-6. [Crossref] [PubMed]
- Yang X, Zhu TY, Wen LC, et al. Intraarticular Injection of Allogenic Mesenchymal Stem Cells has a Protective Role for the Osteoarthritis. Chin Med J (Engl) 2015;128:2516-23. [Crossref] [PubMed]
- Udehiya RK. Comparison of autogenic and allogenic bone marrow derived mesenchymal stem cells for repair of segmental bone defects in rabbits. Res Vet Sci 2013;94:743-52. [Crossref] [PubMed]
- Saito T, Kuang JQ, Bittira B, et al. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg 2002;74:19-24; discussion 24. [Crossref] [PubMed]
- Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001;98:2396-402. [Crossref] [PubMed]
- Bowen CV, Zhang X, Saab G, et al. Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cells. Magn Reson Med 2002;48:52-61. [Crossref] [PubMed]
- Arbab AS, Bashaw LA, Miller BR, et al. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation 2003;76:1123-30. [Crossref] [PubMed]
- Kostura L, Kraitchman DL, Mackay AM, et al. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 2004;17:513-7. [Crossref] [PubMed]
- Arbab AS, Yocum GT, Kalish H, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 2004;104:1217-23. [Crossref] [PubMed]