An update of circulating rare cell types in healthy adult peripheral blood: findings of immature erythroid precursors
Introduction
Various circulating rare cell (CRC) types may exist amongst the main cellular constituents of the blood and in other bodily fluids depending on age and health status. Rarity may account for concentrations lower than 1,000 cells per mL (1-4). The emergence and/or elevation in frequency of certain CRC types may represent benign or more severe pathological conditions such as autoimmunity, immune deficiency and hematologic, cardio-vascular, cancer and other malignancies (2,5-9). Therefore, the detection and quantification of certain CRCs has been used or has potential to be used as diagnostic, prognostic and predictive biomarker (2,10-14) and in non-invasive prenatal diagnosis (15,16). Clinically relevant CRC types may include stem and progenitor cells purposed for tissue regeneration and wound healing (17,18). Other types may include aberrant as well as non-aberrant cells of endothelial, epithelial or mesenchymal origin (2,5-7). Also, cells native to the bone marrow, such erythroblasts and megakaryocytes have been identified (3,19,20).
CRCs that are foreign to peripheral blood are deficient of the common leukocyte antigen CD45 and best enriched by depletion of leukocytes also known as the negative selection principle. Improvement in such technologies enables “mining” for new CRC types in presumption of clinical translation as candidate biomarkers. Left over nucleated cellular fractions of healthy donor blood samples after microchip facilitated red blood cell and CD45 positive cell depletion revealed abundance of bone marrow native erythroblasts, suggesting rarity yet commonness of such erythroid related cell types (3). To date, erythroblasts are routinely identified by blood analysers requiring concentrations above 1×104 cells per mL, which then is commonly associated with severe pathological conditions such as cancer, ischemia and haematological disorders (8).
In this work, we isolated erythroblasts from healthy donors based on the negative selection principle. Our findings allude to the detection of erythroblast-like cells at different stages of maturation. Future work intends to investigate the correlation of frequency and stage of early erythroblasts with health status, as such proving usefulness as prognostic as well as early stage diagnostic biomarker in particular in oncology.
Methods
Blood collection and processing
Blood samples were obtained from PB collected from healthy volunteer subjects using standard 21G’ butterfly needle set. PB was taken by venous puncture collecting 8 mL in green-top BD Vacutainer blood collection tubes containing sodium heparin. The blood sample was processed immediately after phlebotomy or at latest 3 hours after. The study protocol was undertaken as approved by the institutional review board/independent ethics committee of Mahidol University. Informed consent was sought from blood donors at each time.
Red blood cell lysis
Standard chemical lysis buffer treatment was applied to remove red blood cells (RBC) (154 mM NH4Cl, 10 mM NaHCO3, 2 mM EDTA) from 5 mL whole blood adjusting the blood sample to lysis buffer ratio to 1:25 for PB. The cell suspension was incubated at RT for max. Five min and subsequently centrifuged at 300×g for another 10 minutes. The cell pellet was resuspended in 10 mL PBS, supplemented with 0.5% bovine serum albumin and washed by centrifugation at 200×g for 10 minutes. The final cell pellet of nucleated cells with contaminations of platelets and RBCs was resuspended in 1 mL Gibco® Advanced RPMI 1640 and kept at 4 °C. The cell numbers of nucleated cells were determined by hemocytometer (Neubauer) and subjected to experimentation within 1 hour.
Isolation and detection assay
We isolated and detected peripheral blood rare cells from healthy donor PB after RBC lysis by negative selection based on magnetic bead cell separation technology and by fluorescence microscopy. The magnetic bead assay followed descriptions of our previously described dynamic magnetic labeling (DML) procedure (21). In brief, nucleated cell counts were adjusted to maximal 3×107 nucleated cells after RBC lysis from initially 3.5 to 8 mL whole blood. The enrichment procedure followed 2 cycles of DML enrichment using a prototype semi-automated magnetic labelling system (SanoLibio GmbH, Deutschland) and magnetic beads reactive against CD45 (SanoLibio GmbH, Deutschland). After each depletion cycle, uncaptured cell material was pelleted at 300×g for 5 minutes and at room temperature. Subsequent to enrichment, the untouched life cell suspension was stored in 1 mL Gibco®Advanced RPMI 1640 at 4 °C for further use not longer than 1 hour.
For microscopic analysis untouched and enriched life cell suspensions were adjusted to 30 µL and incubated in Gibco® Advanced RPMI 1640 with anti-CD45PE (ebioscience), anti-CD71FITC (ebioscience) and anti-GPAPerCP-C5.5 (ebioscience) each using 1 µL undiluted dye solution in the cold and dark for 20 minutes. Nucleus staining followed using 0.5 µL Hoechst 33342 DNA staining (ThermoFischer). The suspension was washed in 1.5 mL PBS and subsequent centrifugation, 300 ×g 5 min at 4 °C. The pellet was resuspended in 70 µL DMEM Media not containing phenol red (ThermoFischer) and loaded into one well of a specialized 96-well plate suitable for high resolution image recording at 40× magnification using the Operetta system (PerkinElmar) recording a bright field channel, and channels for UV, green, yellow and red fluorescence emission. Columbus analysis software served as screening and image analysis tool. Staining positive cells were identified by a cell-like round formation identifiable by morphology, positive high intensity Hoechst staining and positive FITC fluorescence in the absence of the typical ring formation as consequence of positive CD45PE staining throughout the emission light spectrum from 520 till 650 nm.
Results
We investigated the occurrence of erythroid precursors in peripheral blood that are commonly associated with pathological findings and have been rarely reported for its presence in healthy individuals. Blood donors appeared in healthy condition with respect to feeling, which was further supported by additional hematology data of some of the donors (Table 1). We used our previously reported pan-CD45neg cell enrichment assay based on the DML method for enhanced enrichment of rare cells in the PB by negative selection (21). The pan-CD45neg cell assay allowed an approximate 100× sample volume reduction from a mean blood volume of 4.7 mL (range, 3.5–8 mL). The lower final volume facilitated analysis by automated microscopy image recording in one microplate well per sample, which was convenient for experimental practice and allowed relative short image recording time. Removal of RBCs and high depletion of leukocytes was necessary to avoid cell crowding at the well bottom otherwise rendering image analysis impossible.
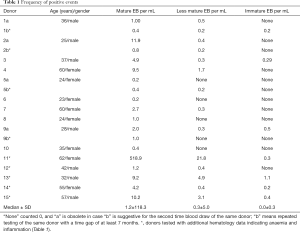
Full table
The negative cell selection principle is independent of marker expression. Therefore, the DML method allows the detection of CD45 negative erythroblasts at any stage of maturation apart from the CD45 positive pro-erythroblast. RBC line specific characterization of the carryover rare cell populations was done by immuno-phenotyping marking CD71 and GPA as positive events. In general, erythroid precursors can be characterized by immuno-phenotyping using a set of cell surface markers that include the transferrin receptor antigen CD71, GPA, Kell blood group protein, integrin associated protein CD47, and the glycoprotein antigen CD44. High expression of CD71 and GPA are unique to cells related to the nucleated red blood cell line (22).
Previous findings would allow the expectation to detect CRCs of the erythroid lineage in the carryover fraction after depletion of WBCs (3). However, detailed morphological distinctions have not been reported so far and positive cell exhibitions included rather small cells not exceeding 10 µm in diameter. Our results show intra and inter donor variation of CD71/GPA positive cells related to the morphological appearance under bright field microscope and the cell and nucleus diameter (Figure 1).
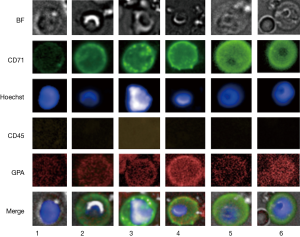
The assumption seems likely of distinct stages of cell maturation that may include basophilic erythroblasts (BasoE), polychromatophilic, erythroblasts (PolyE) and orthochromatic erythroblasts (OrthoE). Fluorescence staining protocols have been applied to erythroblast staging using cell and nuclear size as well as fluorescence signal intensities characteristics (23,24). The expression of the transferrin receptor CD71 is generally expected to decrease and nucleus intensity to increase with progress of maturation, due to progressive nuclear condensation. However, the interpretation is not without pitfalls. The CD71 correlation with maturation in particular for stages between BasoE and OthoE are not stringently reproducible (23). Also, in case of PB circulating erythroblasts, marker expression, mode of differentiation and cell viability may be altered over time as a result of dwelling in an environment other than the bone marrow. Furthermore, the small number of in particular larger cells does not provide statistical significance. In view of the uncertainties, classical or detailed staging of PB circulating erythroblasts may be difficult. Therefore, we group positive events coarsely into immature, less matured and matured erythroblasts according to bright field appearance, cell and nuclear size (Figure 1). Thus, immature erythroblasts would include cells with cell and nucleus size larger than 13.5 and 6.5 µm in diameter, respectively as shown in Figure 1, rows 4 to 6. Less mature EBs would be larger in either or both cell or nucleus size, thus ranging from 10 till 13.5 µm and 6.5 till 8 µm, respectively and illustrated in Figure 1 rows 2 and 3. The smallest cells are expected to have reached a final stage of maturation with cell and nucleus size below 10 and 6.5 µm, respectively. The results suggest the idea of EB maturation stage profiles and show that rarity increases with cell size, thus cell immaturity (Table 2). Inflammation as indicated by the C-reactive protein concentration was not correlated with appearance of in particular immature erythroblasts. Donor 11 stands out with a high concentration of in particular mature erythroblasts that may be correlated with a stable and asymptomatic condition of slight anemia and/or an underlying condition of thrombocytosis (7.8×105 platelets per uL). The presence of in particular less mature and immature EBs in the circulation is commonly expected to be disease- and disease stage related (8,20). Recent work has revealed nucleated red blood cells to possess functionalities similar to innate as well as adaptive immune cells in trout (25) and chicken (26), as such being players in inflammation and infection (27).
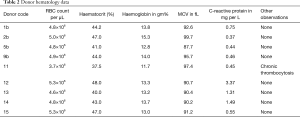
Full table
Conclusions
In view of the general donor healthiness on grounds of feeling and hematology data, our findings of immature erythroblasts may represent a benign baseline concentration. We may hypothesize that the assessment of the EB profile as proposed in this work could be a useful biomarker for health status identification in general, early stage disease detection, such as in situ tumor growth or cancer therapy monitoring and response.
Acknowledgements
The authors are grateful to all blood donors and in particular to Teerapat Rodboon of the Faculty of Science, Mahidol University for conducting phlebotomy and supporting lab analysis. This research project was funded by the Mahidol University Postdoctoral program and in parts by the Thailand Center of Excellence in Physics.
Footnote
Conflicts of Interest: We would like to report that Stefan Schreier has a business interest in reporting the use of SanoLibio cell enrichment technology, a company that may be affected by the research reported in the enclosed paper. We have disclosed those interests fully to Ann Transl Med and have in place an approved plan for managing any potential conflicts arising from that involvement.
Ethical Statement: The study was approved by the Institutional Review Board/Independent Ethics Committee of Mahidol University (IRB number: 2016/032.2103). Informed consent was sought from blood donors at each time.
References
- Hyun KA, Jung HI. Microfluidic devices for the isolation of circulating rare cells: a focus on affinity-based, dielectrophoresis, and hydrophoresis. Electrophoresis 2013;34:1028-41. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Fachin F, Spuhler P, Martel-Foley JM, et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci Rep 2017;7:10936. [Crossref] [PubMed]
- Castle J, Morris K, Pritchard S, et al. Challenges in enumeration of CTCs in breast cancer using techniques independent of cytokeratin expression. PLoS One 2017;12. [Crossref] [PubMed]
- Pantel K, Denève E, Nocca D, et al. Circulating epithelial cells in patients with benign colon diseases. Clin Chem 2012;58:936-40. [Crossref] [PubMed]
- Yang C, Zhuang W, Hu Y, et al. Clinical significance of peripheral circulating tumor cell counts in colorectal polyps and non-metastatic colorectal cancer. World J Surg Oncol 2018;16:13. [Crossref] [PubMed]
- Li Y, Liu J, Zhao Z, et al. Correlation between circulating endothelial progenitor cells and serum carcinoembryonic antigen level in colorectal cancer. Acta Biochim Biophys Sin 2018;50:307-12. [Crossref] [PubMed]
- Danise P, Maconi M, Barrella F, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med 2011;50:357-60. [PubMed]
- Hiraiwa K, Takeuchi H, Hasegawa H, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol 2008;15:3092. [Crossref] [PubMed]
- Purtle SW, Clare MH, Moromizato T, et al. Nucleated red blood cells, critical illness survivors and postdischarge outcomes: a cohort study. Crit Care 2017;21:154. [Crossref] [PubMed]
- Groen J, Godfried EG. The occurrence of normoblasts in the peripheral blood in congestive heart failure: An indication of unfavorable prognosis. Blood 1948;3:1445-52. [PubMed]
- Menk M, Giebelhäuser L, Vorderwülbecke G, et al. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann Intensive Care 2018;8:42. [Crossref] [PubMed]
- Stachon A, Segbers E, Holland-Letz T, et al. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Crit Care 2007;11:R62. [Crossref] [PubMed]
- Desai S, Jones SL, Turner KL, et al. Nucleated red blood cells are associated with a higher mortality rate in patients with surgical sepsis. Surg Infect (Larchmt) 2012;13:360-5. [Crossref] [PubMed]
- Byeon Y, Ki CH, Han KH. Isolation of nucleated red blood cells in maternal blood for Non-invasive prenatal diagnosis. Biomed Microdevices 2015;17:118. [Crossref] [PubMed]
- Zheng YL, Carter NP, Price CM, et al. Prenatal diagnosis from maternal blood: simultaneous immunophenotyping and FISH of fetal nucleated erythrocytes isolated by negative magnetic cell sorting. J Med Genet 1993;30:1051-6. [Crossref] [PubMed]
- Lo Sicco C, Tasso R, Reverberi D, et al. Identification of a new cell population constitutively circulating in healthy conditions and endowed with a homing ability toward injured sites. Sci Rep 2015;5:16574. [Crossref] [PubMed]
- Ciraci E, Della Bella S, Salvucci O, et al. Adult human circulating CD34− Lin− CD45− CD133− cells can differentiate into hematopoietic and endothelial cells. Blood 2011;118:2105-15. [Crossref] [PubMed]
- Buoro S, Vavassori M, Pipitone S, et al. Evaluation of nucleated red blood cell count by Sysmex XE-2100 in patients with thalassaemia or sickle cell anaemia and in neonates. Blood Transfus 2015;13:588-94. [PubMed]
- Buoro S, Manenti B, Seghezzi M. Which clinical significance has automatic detection of very low levels of nucleated red blood cells in the peripheral blood? Ann Transl Med 2016;4:230. [Crossref] [PubMed]
- Schreier S, Sawaisorn P, Udomsangpetch R, et al. Advances in rare cell isolation: an optimization and evaluation study. J Transl Med 2017;15:6. [Crossref] [PubMed]
- Dong HY, Wilkes S, Yang H. CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: a comparative immunochemical study with glycophorin A and hemoglobin A. Am J Surg Pathol 2011;35:723-32. [Crossref] [PubMed]
- McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: where flow meets morphology. J Immunol Methods 2008;336:91-7. [Crossref] [PubMed]
- Thanuthanakhun N, Nuntakarn L, Sampattavanich S, et al. Investigation of FoxO3 dynamics during erythroblast development in β-thalassemia major. PLoS One 2017;12. [Crossref] [PubMed]
- Puente-Marin S, Nombela I, Ciordia S, et al. In Silico Functional Networks Identified in Fish Nucleated Red Blood Cells by Means of Transcriptomic and Proteomic Profiling. Genes 2018.9. [PubMed]
- St Paul M, Paolucci S, Barjesteh N, et al. Chicken erythrocytes respond to Toll-like receptor ligands by up-regulating cytokine transcripts. Res Vet Sci 2013;95:87-91. [Crossref] [PubMed]
- Nombela I, Ortega-Villaizan M. Nucleated red blood cells: Immune cell mediators of the antiviral response. PLoS Pathog 2018;14. [Crossref] [PubMed]


