Solid organ transplantation in the 21st century
Introduction
Solid organ transplantation (SOT) has transformed the survival and quality of life of patients with end-organ dysfunction. SOT offers life-saving treatment for diseases considered terminal or those associated with a significant impairment in a patients’ quality of life. The evolution of SOT is marked by technical advancement, pharmacologic development, innovation in broadening the donor pool, and the standardization of practices related to transplantation. The current volume of SOT performed in the United States is summarized in Figure 1.
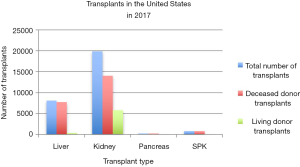
The modern era of SOT saw its inception in the early 20th century with the application of successful techniques on humans after decades of methodological refinement in animal models. The development of a novel concept, the technique of vascular anastomosis and suture techniques, is attributed to the work of Alexis Carrel, Mathieu Jaboulay, and Julius Dorfler (1,2). Using the techniques initially described by Carrel, Ullmann described the first technically successful kidney autotransplants performed in dogs and dog-to-goat xenografts (3). Jaboulay later performed the first renal xenotransplants in humans in 1906, using a pig donor in one patient and a goat donor in a second patient (2). Both xenografts failed and both patients subsequently died (2). Other experimental transplantation of the thyroid, ovary, heart, lung, and small bowel in animals and humans were also performed around this time by Carrell and others around the world.
SOT was now possible at this point, however the solid organ graft survival and function required improvement. Emerging from the initial experimental SOT procedures was the recognition that autografted tissue (such as skin grafts) could be successfully transplanted without immediate graft loss, but homografts of both skin and solid organs consistently failed over a short period of time (2). Additionally, in 1954, it was reported that a kidney transplant between identical twins was successful and achieved long term function and resolution of the patient’s malignant hypertension (4). It was these experiences and other experimental models (5) that provided a foundation for discovering critical immunologic concepts for transplantation, including the basis for allograft rejection and the development of immunosuppressive pathways for future therapy. Medawar first described the need for immunosuppressive therapy to achieve sustained graft function. The first attempts at immunosuppression involved radiation for depletion of the immune response. In 1955, Main and Prehn demonstrated that immunosuppression through radiation improved the success of bone marrow transplantation. Shortly thereafter in 1958, improved renal graft function in kidney transplant recipients was noted when recipients were subjected to thiopurine or total body irradiation before the transplantation procedure, albeit with limited long-term success (6,7).
Pharmacological means of immunosuppression were also attempted at this time. In the 1950’s and 1960’s, 6-mercaptopurine (6-MP), its derivative azathioprine, and nitrogen mustard were implemented for the purpose of transplant-related immunosuppression. In 1960, Calne showed that 6-MP significantly prolonged the survival of dogs who received dog kidney homografts (8,9). Thomas Starzl, the father of modern surgical transplantation, presented an immunosuppressive protocol in 1963 that allowed for a 70% 1-year renal graft survival (10). The associated immunosuppression regimen involved prednisone and azathioprine (10). Subsequent developments such as antilymphocytic serum, dialysis, antibody screening, HLA typing, donor organ protocols, and organ preservation led to further improvement in the success of SOT. By 1963, the first human liver transplant was performed along with the use of an early immunosuppression protocol (10). In 1964, the first successful human heart transplant was performed (11). Pancreatic and lung transplantation occurred soon thereafter in 1966 and 1987, respectively (12,13).
The national acceptance of the concept of “brain death” in 1968 marked another advancement in the field of transplant. Brain death is considered to be an irreversible cessation of brain function and indicates that the patient is clinically and legally deceased. The three main criteria for brain death are coma, absence of brainstem reflexes, and apnea (14). Donation after brain death (DBD) organs shows a lower risk of ischemia as compared to donation after circulatory death (DCD) (15) because DBD organs are physiologically well perfused at the time of procurement. The availability of donors after brain death has led to a greater number and wider variety of organs available for SOT beyond the confines of a single hospital system. This led to the formation of the Organ Procurement and Transplantation Network (OPTN) to oversee the regional and national allocation of organ resources. The field of transplant continues to evolve. The 21st century has ushered in a world of reliable and effective SOT, as well as unconventional transplantation of other tissue such as upper extremity, uterus, larynx, and face. The aim of this paper is to review the current state of SOT in commonly transplanted organs and discuss recently developed techniques and innovations.
Liver transplant
The early history of liver transplantation (LT) alone illustrates how innovation can revive and legitimize a field that was once all but condemned. Starzl’s first attempts of LT in 1963 were met with failure. Patients died either during or shortly after the operation (16). Starzl and Calne, recognized that early setbacks included tissue ischemia, immunosuppression, coagulopathy challenges regarding biliary reconstruction, and ultimately a reflection of un-refined surgical technique (16). By the late 1960’s and 1970’s, some patients were surviving longer than 1 year following LT with early immunosuppression protocols (16). The ensuing 50 years of medical and surgical advancement would result in LT yielding 1- and 5-year survival of 91.2% and 75%, respectively (17). LT is the accepted definitive treatment modality for an array of etiologies leading to end-stage liver disease (ESLD) such as cirrhosis, nonalcoholic steatohepatitis, hepatocellular carcinoma, and other metabolic diseases (18).
Allocation
Because the demand for LT exceeds the organ supply, organized systems of organ allocation are required for an objective and equitable distribution of livers. In the 1990s, there was a shift from emphasis on waiting time and subjective patient care parameters to implement objective tools such as the Child-Turcotte-Pugh (CTP) score to fairly allocate organs (19). Historically, the CTP was correlated with mortality risk and disease severity to triage patients for LT (19). With time, the transplant community transitioned to the Model for End-Stage Liver Disease (MELD) score in 2002 and this remains the standard for transplant allocation scoring. The MELD score is based on serum creatinine, bilirubin, international normalized ratio (INR) of prothrombin time, and takes into account special scenarios related to etiology of liver disease. The MELD score has also been shown to accurately predict the 3-month mortality of a patient without a transplant. It was modified in 2006 to include exception criteria, which made it more inclusive for patients whose urgent need for a LT was not appreciated with the original scoring system (16). A Pediatric End Stage Liver Disease (PELD) score was also developed for children, which currently includes age, albumin, INR, and growth failure. The scoring methods have helped to more equitably allocate livers. However, due to the strain of organ demand, the field has seen an increase in higher risk or extended criteria donors (including steatotic livers, older organs, and those with positive serology for viral pathology) as well as DCD (20).
Surgical technique
The first liver transplants in the 1960s educated the field on the extremes of bleeding coagulopathies, portal hypertension, and significant hemodynamic instability associated with taking ESLD patients to the operating room. Countless advances in surgical technique, intraoperative care, and peri-operative intensive care have enhanced patient survival and longevity of the donor liver. Examples include advanced intraoperative anesthesia monitoring, aggressive coagulopathy prevention and treatment, the development of tailored retractors for optimal exposure of the vasculature and biliary system, and hemostatic surgical devices. Though inherently challenging, yet significantly safer today than in the early days of transplantation, the hepatectomy of the donor liver is the initial surgical procedure. The majority of donor transplants today are procured from deceased donors (82%) while the rest are attained from living donors (17). Surgical technique varies depending on the status of the donor, however, liver procurement from a deceased donor often occurs in conjugation with other organs including kidney, pancreas, heart and lung procurement. The dissection of the deceased donor liver can be carried out through the traditional technique or the rapid technique. Both techniques pay careful attention to preserving integrity and blood supply of the organ and utilize rapid core cooling. The rapid technique has been implemented in multi-organ retrieval in unstable donors due to its rapid procurement time but requires a greater degree of skill (21).
Following procurement of the donor liver, the recipient undergoes hepatectomy of the diseased liver. Hepatectomy is performed with either the conventional technique or the piggyback technique. The conventional technique (also known as the bicaval technique) involves removing the recipient retro-hepatic inferior vena cava (IVC) en bloc with the liver, allowing for anastomoses of the donor liver vena cava in the supra- and infra-hepatic portions of the IVC (22). In the piggyback technique, the recipient liver is completely dissected from the IVC, leaving a short recipient hepatic vein cuff that can be anastomosed to the donor suprahepatic IVC. This offers less hemodynamic changes associated with the transient occlusion of the native IVC and a decreased anhepatic portion of the procedure while also minimizing warm ischemia times with fewer anastomoses (22). Veno-venous bypass can be used during the anhepatic phase in the conventional method to ensure continuous venous return as well, however, this approach is less commonly employed.
The next step of the operation involves portal vein anastomosis and restoring hepato-portal reperfusion. The donor liver is flushed with saline which may be followed by warm recipient blood to mitigate the risks of reperfusion syndrome (22). The surgeon will also perform a portal vein thrombectomy if a clot is present in the donor liver (22). The clot is usually identified on pre-operative imaging of the donor liver, allowing the surgeon to plan for the thrombectomy. The next anastomosis is of the hepatic artery. The most common approach is to create an anastomosis between the donor’s celiac artery and the recipient’s common hepatic artery though variations may be used on a case-by-case basis depending on arterial diameter discrepancy, aberrant anatomy, vessel length, and tension of the anastomosis. Lastly, the biliary anastomosis is performed between the donor and recipient common bile ducts (22). If the duct size is mismatched or technically challenging, the surgeon can perform a hepaticojejunostomy with a Roux-en-Y intestinal reconstruction (22).
Split-liver donor/living donor liver transplants (LDLT)
A major advancement in hepatic transplantation was split-LT (SLT) and LDLT in 1988 (23). Both techniques serve to broaden the supply of transplantable liver tissue.
SLT refers to division of the donor liver from a deceased adult, yielding two viable organs from one donor. Most commonly, the donor liver is split such that a larger portion (right tri-segment allograft) can go to an adult patient and a smaller portion (left lateral segment allograft) can be allotted to a pediatric patient. Therefore, SLT has especially befitted the ESLD pediatric population. Alternatively, the donor liver can be split in a configuration for two adult recipients; however, this is controversial and not as commonly performed (24,25). The split liver technique for two adult recipients remains experimental because recipient and donor liver survival outcomes are not comparable to conventional whole liver transplants. However, ongoing studies show potential in this strategy and future trials may reveal a more accurate pairing by using donor-to-recipient weight ratio to better predict donor liver function and longevity with regard to transplant recipient hepatic needs (25).
Similarly, LDLT is the transplantation of a partial graft of a living donor’s liver. Due to cultural views relating to transplantation of deceased organs, the development of LDLT has been particularly of interest in Asia. Currently, the risk of donor morbidity is approximately 20% and mortality is 0.5% (16,26). Risk of donor morbidity and mortality must be minimized to allow such techniques to gain more acceptance and be offered to a broader subset of patients. LDLT is a technically challenging procedure and is associated with several recipient complications including hepatic arterial stenosis and biliary complications (27-29). Examples of biliary complications include strictures, anastomotic leaks, choledocholithiasis, cholangitis, etc. (27). A recent meta-analysis showed that, despite higher rates of complications, a larger pool of patients is able to receive a lifesaving partial LT with no significant change in long term survival when compared to deceased donor orthotopic LT (30). The study excluded SLT (30).
Both SLT and LDLT help meet the exceeding need of livers while attempting to minimize unfavorable post-transplant outcomes (31,32). Ongoing data continues to suggest that adult and pediatric SLT and LDLT have comparable patient and graft survival to whole liver transplants (25,32).
Kidney transplant
History
The first successful human kidney transplant occurred between identical twins at the Peter Bent Brigham Hospital in Boston in December of 1954. Kidney transplantation is now routinely performed as the treatment of choice to improve quality of life and survival for patients with end-stage renal disease (ESRD). Etiologies of ESRD include but are not limited to diabetic nephropathy, longstanding hypertension, and various types of nephrotic and nephritic diseases. Kidney transplantation has shown to improve survival as compared to patients on dialysis (33).
Deceased donor kidney transplants (DDKT) vs. living related donor kidney transplants (LRDKT) and graft survival outcomes
Currently, 30% of donors are LRDKT and 70% are DDKT (18). LRDKT organ donors are usually family members or close friends to the patient, although altruistic non-directed kidney donations exist as well. The impact for living donors is not insignificant (34). Living kidney donation is associated with an increased risk of gestational hypertension, pre-eclampsia, and rarely ESRD (<0.5% increase in incidence at 15 years) (35). However, living donors in the U.S. have a similar life expectancy and quality of life as the average healthy patient who does not donate a kidney (35).
Living transplants are associated with better graft and recipient patient survival (36). The most recent national data from the U.S. Department of Health and Human Services shows that 1-, 3-, and 5-year survival rates are 97.5%, 92.6%, and 85.6%, respectively, for LRDKT and 93.2%, 85.1%, and 74.4%, respectively, for DDKT (17). Interestingly, the number of LRDKTs decreased dramatically over the past decade (37). For instance, the rate of pediatric LRDKTs comprised 47.2% of pediatric kidney transplants in 2005 and only 34.2% in 2016 (37). While 2015 to 2017 saw an increase in the number of kidney transplants performed altogether, the increase was mostly attributable to an increase in DDKT (37). Research has been done to investigate the reason for the decline in LRDKT, citing financial disincentives, declining health status of the general population (increased prevalence of obesity, diabetes, and hypertension), shorter wait times and increase number of donations of DDKT kidneys (38).
Allocation of kidneys
Guidelines from the National Kidney Foundation provide useful information on which patients qualify for kidney transplant based on various factors such as stage of kidney disease, cardiovascular status, and presence or absence of chronic infection (39,40). In general, any patient approaching stage 4 chronic kidney disease or end-stage renal disease should be evaluated for inclusion on the kidney transplant listing, even if the patients are not yet on hemodialysis. Kidneys are then allocated to those on the transplant waiting list via the Kidney Allocation System (KAS), which was started in 2014. KAS matches donor kidneys with the longest potential graft survival to the patient that has the longest life expectancy. Donor kidneys are given a kidney donor profile index (KDPI) score. This score summarizes the likelihood of graft survival after DDKT (41). Lower KDPI scores are associated with longer graft survival and higher scores are associated with shorter duration of kidney graft function (41). Once offered, the patient can then decide if they will accept the kidney based on their health status, quality of life, and transplant center logistics. This becomes especially relevant for high risk donor organs such as donors with a history of incarceration, intravenous drug use, risky sexual behavior, or even hepatitis or HIV status if the recipient has a positive history as well. The KAS system has been successful at providing equitable distribution of kidney transplants, which has improved the number of transplants in patients with a lower socioeconomic status since its inception in 2014 (37).
Alternatively, some kidney transplant patients who are not yet on dialysis and ranked non-urgently on the transplant list may consider pre-emptive transplantation (42). Pre-emptive transplantation typically occurs in the setting of ESRD and occurs in the context of a LRDKT and is associated with superior allograft survival and patient survival (42).
The concept of “matched pair donation” was developed by Segev et al. to increase transplant opportunities for living donor and recipient pairs who are blood-type or cross-match incompatible (43). The concept of matched pair donation refers to a process where a living donor who fails to match to their intended recipient can donate an incompatible organ within a national system and the recipient can therefore match with another incompatible living donor-recipient mismatch pair (43,44). As a result, both people in need of a transplant will receive a compatible organ. This system is organized under the National Kidney Paired Donation (KPD) program (43). Pooled living donor donation has improved wait times in most situations, although O blood type recipients still experience longer wait times than their AB counterparts (43).
Surgical strategy
Living related donor kidney allografts are usually retrieved laparoscopically or laparoscopic-assisted. Laparoscopy has made living donation less morbid by reducing incision size, reducing postoperative pain, decreasing narcotic requirements, and decreasing the length of hospital stay (45).
The most common surgical approach for transplanting the donor kidney into the recipient patient is to implant the kidney extraperitoneally in the pelvis. Three anastomoses are typically performed which include the arterial anastomosis usually between the donor kidney renal artery and the recipient’s right external iliac artery, the venous anastomosis between the donor kidney renal vein and the recipient’s right external iliac vein, and lastly, the donor ureter is anastomosed to the recipient bladder (44). A stent is commonly placed across the ureterovesical anastomosis and removed 8–12 weeks post-transplant (44). Typically, a LRDKT allograft is expected to function immediately following allograft reperfusion or following the operation (18,36). However, up to 40% of DDKT allografts do not function immediately (18). This phenomenon is called delayed graft function (DGF) and has been reported to occur in 21.3% of cases (46). DGF is the development of acute kidney injury (AKI), requiring dialysis within 7 days of the transplant (46). Once technical issues have been addressed and graft rejection ruled out, the patient is managed with hemodialysis until the AKI resolves (18). Allograft dysfunction of the DDKT may occur up to 1–2 months after transplant (18). DGF can have a clinically significant impact on early graft survival. The 1-year graft survival of DDKT with DGF is significantly lower (76.3%) compared to DDKT with no DGF (92.3%) (47). Interestingly, both groups share a similar graft half-life of roughly 20 years. However, DGF is often confounded by acute rejection which can then severely diminish graft 1-year survival (67.9%) resulting in a half-life of 10.5 years (47). Therefore, while DGF can complicate early post-transplant management, the long-term outcomes of DDKT with DGF are negligible unless the etiology of DGF results in subsequent acute rejection.
Pancreas transplant
History
The purpose of a pancreas transplant is to provide or restore beta cell function to a diabetic recipient. The first successful human pancreatic transplant was performed in 1966 by Drs. Kelly and Lillehei at the University of Minnesota (48,49). Since its conception over 50 years ago, pancreas transplantation has evolved into the definitive intervention for hormone replacement therapy to restore normoglycemia in those with diabetes mellitus (48,49). Although the first successful pancreas transplantation occurred in the late 1960s, the initial investigations date back to the 1890s with initial pancreatic transplant animal model (48-50). The past 50 years have ushered in various strategies for optimizing pancreas transplant outcomes, specifically surgical techniques and immunosuppression medications (49).
Surgical strategies
All pancreatic transplantations are from deceased donors, either DBD or DCD. Grafts from either DBD or DCD have similar survival rates (51). Currently, there are four types of pancreatic transplantation options that are available. The first surgical approach is a pancreas-alone transplant (PAT). This type of transplant is recommended for diabetics with frequent episodes of hypoglycemia (i.e., type 1 diabetes mellitus, low quality of life, and/or noncompliance with insulin therapy) (48). Patients eligible for PAT should have adequate renal function (glomerular filtration rate of 80–100 mL/min/1.73 m2) with no evidence of uremia (48). However, PAT would not be the ideal surgical option in a patient who has diabetic nephropathy. For these patients, the more effective surgical approach is the simultaneous pancreas-kidney (SPK) transplant. Both kidney and pancreas are obtained from a single deceased donor and transplanted into the recipient patient. SPK transplant is associated with a lower rate of immunologically mediated graft loss compared to PAT (48,49). Patient and graft survival rates following SPK transplantation are comparable to those for kidney transplantation alone (52).
Another option includes the pancreas-after-kidney transplant (PAK). This involves a living donor kidney transplant followed months later by a deceased donor pancreas transplant (18). This may be the ideal operation if a living donor kidney transplant is available, therefore reducing the time the patient would need to be on dialysis while waiting on the deceased donor list for a pancreas transplant. However, the risk of two genetically unique transplanted organs in one recipient increases the risk of acute and chronic rejection. Additionally, PAK involves two separate operations instead of one. Therefore, in most cases, SPK is the preferred procedure (18). Simultaneous transplantation of a deceased donor pancreas and living donor kidney (SPLK), is a modification of the PAK approach, which attempts to minimize the risk associated with two operations. This is predicated on the patient’s living donor’s willingness to be readily available as soon as a deceased pancreas donor becomes available.
Regardless of the operative approach, all pancreatic transplantation involves three main anastomoses: venous, arterial, and exocrine drainage through the duodenum or bladder. For venous drainage, the donor pancreas is harvested en bloc with the attached portal vein, which is subsequently anastomosed to the recipient vena cava, iliac vein, or superior mesenteric vein (53). The arterial anastomosis involves the harvest of a donor common iliac artery which is anastomosed to the stumps of the donor splenic artery and superior mesenteric artery (SMA), and the other end of the donor common iliac artery is anastomosed to the recipient common iliac artery (18).
For exocrine drainage, the surgeon can choose between enteric drainage or bladder drainage. Enteric drainage involves an anastomosis between the duodenum segment of the donor pancreas to the recipient small intestine (54,55). The enteric anastomosis was the gold standard in the earlier days of transplantation. The field saw a shift in the 1990’s towards bladder anastomosis and drainage. In this technique, the duodenal segment of the graft is anastomosed to the dome of the recipient bladder. This technique gained popularity because it allowed for clinicians to monitor urinary lipase and amylase to better assess for early signs of graft rejection and provide earlier opportunities for early intervention (54,55). Bladder drainage is not without adverse consequences and places patients at risk for urologic and metabolic complications such as urinary tract infections, metabolic derangements, dehydration, hematuria, reflux pancreatitis, and bladder stones (55). In the face of severe complications, patients can undergo a conversion procedure where the pancreas can be converted to enteric drainage (55). Several retrospective studies demonstrated no significant difference in 1-year graft survival between the two ductal drainage techniques (54-56). To date, there are no randomized controlled trials supporting a specific type of exocrine drainage, thus the approach is left to the discretion of the surgeon and institution and is determined on a patient-specific basis (55).
Outcomes of pancreas transplant
One-year pancreas allograft survival rates have reached 90% with SPK transplantation, 83% in PAK transplantation, and 80% with PAT (57). An analysis of 18,159 pancreas transplants from the International Pancreas Transplant registry showed that 5-, 10-, and 20-year graft survival rates were 80%, 68%, and 45%, respectively, for SPK, 62%, 46%, and 16% for PAK transplants, and 59%, 39%, and 12%, respectively, for PAT (58).
Islet cell implantation and future directions
In recent years, there has been a growing desire to use islet cell transplantation as an alternative to whole-organ pancreatic transplantation. Islet cell transplantation is a much simpler procedure, and would theoretically avoid the complications of a SOT while restoring endogenous pancreatic function. Islet cell transplantation occurs by isolating islet cells from a deceased donor pancreas using enzymatic digestion. The separated cells are then injected through the portal or mesenteric vein and therefore embolize through the portal venous system to seed the liver (18,59). Upon successful cell implantation, insulin and other pancreatic hormones are subsequently secreted. The procedure allows for broader donor criteria because of the nature of transplanting functioning cells rather than an entire organ and associate vasculature which is susceptible to the litany of vascular and environmental insults. Nevertheless, islet cell transplantation also requires immunosuppression.
The initial experimental work on islet transplantation occurred in the 1970s with the procedure proving to be successful on rodent models (60). The first human clinical trials were conducted in the 1980’s and were largely unsuccessful because of inadequate immunosuppression and technical difficulties (59). The 21st century found more success with the development of the Edmonton Protocol (61). The key elements of the Edmonton Protocol that contributed to the success of islet cell transplantation were the introduction of steroid-free immunosuppression and the use of larger numbers of islet cells (57,61). The Edmonton Protocol implemented immunosuppression agents such as sirolimus, low-dose tacrolimus, and daclizumab (61). The purpose was to eliminate the need for glucocorticoids which are associated with insulin-resistance (61,62). Secondly, the protocol nearly doubled the number of islet cells that were prepared for implantation. Previous studies used 6,000 cells, while the Edmonton Protocol called for the use of 10,000 cell equivalents per kilogram (61,62). The Edmonton Protocol has since undergone various modifications yielding multiple iterations that vary by institution (61,63).
The outcomes of patients who have undergone islet cell transplantation have shown promising results. For example, in 1999 under the support of the Juvenile Diabetes Research Foundation, the collaborative islet cell transplantation registry (CITR) was created which sought to evaluate progress of pancreatic and islet cell transplantation from more than 40 centers in the United States and Canada (64). The aim of this registry is to monitor success of transplantation by primarily assessing insulin independence rates (64). Since the conception of the registry, islet cell transplantation performance has dramatically improved (64). Five-year insulin independence rates in a single donor islet transplant has reached 50% (63,64). Islet cell transplantation is available at many institutions, but not provided as universally as whole-organ pancreatic transplantation. One of the issues that prevent the widespread use of islet cell transplantation is low availability of deceased donor organs to provide for a high number of cells needed for the procedure (59). Additionally, the procedure still requires immunosuppressive therapy, providing the same immunologic challenges as a whole-organ transplant (64).
Immunosuppressive therapy
Rejection remains one of the most feared complications of any SOT. Transplant patients are typically put on life-long immunosuppression protocols to prevent allograft rejection. The goal of various immunosuppression regimens is to prevent the proliferation and cytotoxic actions of T cells while also suppressing antibody production from B cells. Immunosuppression protocols can be divided into induction regimens, maintenance therapy, and rejection treatment. Induction regimens are initiated prior to or immediately during the transplant operation. Typically, high-dose steroids and antithymocyte globulin, alemtuzumab, or basiliximab is used (18). Maintenance therapy is usually a combination of two to three immunosuppressants from separate classes (18) such as tacrolimus, mycophenolate mofetil, and a corticosteroid. Immunosuppression is associated with an increased risk of viral and bacterial infection including CMV, herpes simplex virus, BK polyoma virus, Tuberculosis, Pseudomonas, Pneumocystis carinii, Toxoplasma gondii, Candidiasis, Aspergillus, Nocardia, as well as endemic fungi (histoplasmosis, cryptococcosis, coccidioidomycosis, etc.). Many patients are put on long-term trimethoprim-sulfamethoxazole prophylaxis therapy to prevent a devastating pneumonia from Pneumocystis carinii. Additional increased risks include skin cancer, lymphoma, and cervical cancer and development of significant metabolic derangements.
Future directions
Advances in the field of SOT over the past 60 years have been revolutionary to patient survival. Much of the current research attempting to expand the field focuses on immunomodulation. Immune rejection is mediated by complex signaling molecules, cellular immunity, and humoral mechanisms. Future directions of transplant research focus on attempting to minimize the immune response by inhibiting aspects of these various mechanisms. An example of an immunomodulatory target is the Notch signaling pathway (65). The Notch pathway is a cell-to-cell communication cascade that plays an important role during T cell development and in the regulation of innate lymphoid cells, B cells, and dendritic cells (66). The signaling pathway is a central mediator of T cell reactivity to allografts (65). Recent studies have shown that inhibition of the Notch pathway in animal models can reduce both allograft rejection (67) and graft vs. host disease (68).
Other immune-related advancements in the field of transplant attempt a more targeted inhibition of the immune system. The ideal situation in organ transplant is operational tolerance which is defined as a recipient having immunologic tolerance to the foreign organ in the absence of immunosuppressive drugs (69). With operational tolerance, patients forgo the need for maintenance immunosuppression and the complications associated with such medications are abrogated, including life-threatening infections and increased risk of neoplasms (69). Donor specific tolerance offers the ability to eliminate or minimize the need for immunosuppressive medications. The introduction of hematopoietic stem cells has shown promise in select human cases and many animal models. Initial pilot trials have shown variable yet promising results (70-73). Spitzer et al. demonstrated that three of seven patients receiving a simultaneous bone marrow transplant with a kidney transplant were able to achieve complete immunosuppressive drug withdrawal (70). In another study, seven out of ten patients who developed chimerism via kidney and bone marrow HLA-mismatched transplants achieved immunosuppression freedom for 4.5–11.4 years (71). Scandling et al. demonstrated persistent chimerism (greater than 6 months) resulting in complete withdrawal of immunosuppressive drugs in 16 out of 22 patients (73). Even though the results of such pilot human trials are promising, their adoption and replicability have yet to be proven.
Another method being explored to induce tolerance is through the use of biomaterial carriers (74). Micro and nanomaterial carriers may potentially act as immunomodulating scaffolds that interact with and affect antigen presenting cells, T cells, B cells, and other components of the immune system (74). Lewis et al. showed that microparticles (specifically, poly lactic-co-glycolic acid microparticles) carrying combinations of immunosuppressive drugs may play a role in conditioning dendritic cells to suppress allogenic T cell proliferation, thereby inducing tolerance (75).
Another promising area of research in the field of SOT attempts to counteract the shortage of available human organs for donation. Xenotransplantation, or the transplantation of animal organs into a human recipient, is a growing area of research and has seen some promise in recent years (76). Islet cells, hearts, livers, lungs, and kidneys are all being studied as potential transplantable porcine organs in humans. Initially, xenotransplantation had been met with great resistance regarding concerns of innate and humoral-mediated hyperacute rejection, as well as the potential for transmitting porcine endogenous retrovirus (PERV) (77). Recent advances in gene editing using the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system have decreased the concern of incompatible cross-matching between porcine and human components (76). CRISPR/Cas9 can knock out multiple genes encoding the antigens targeted in an immune response. The technique can also knock genes into the pig genome (78). The CRISPR/Cas9 can also be used to breed PERV knock out pigs to mitigate the challenges of xenotransplantation PERV transmission (79). Additionally, Human immunodeficiency virus drugs such as raltegravir, dolutegravir, and zidovudine have been studied and shown promise in preventing PERV transmission from porcine to human systems (80,81). This approach has yet to enter the clinical arena of transplantation.
SOT has become the standard of care for thousands of patients and offers definitive long-term treatment to patients with otherwise limited options. Transplant research has remained a source of constant innovation and has helped to improve outcomes for patients over time. Improvements in surgical and clinical outcomes have led to the expansion of the field, however, with new advancements come new obstacles to overcome. Of note, increasing donor demand with supply shortage, shifting eligibility and transplantation protocols, long-term graft survival, and immunosuppression-related complications are of major concern in the future of SOT. The greatest hope for overcoming these barriers comes from promising research on expanding the pool of organ candidates, whether via human graft or xenograft, and immunological modifications for improved graft-survival. Advances in the field continue to augment the lives of many afflicted with life-threatening illness.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Carrel A. The transplantation of organs: a preliminary communication. 1905 (classical article). Yale J Biol Med 2001;74:239-41. [PubMed]
- Barker CF, Markmann JF. Historical overview of transplantation. Cold Spring Harb Perspect Med 2013;3. [Crossref] [PubMed]
- Ullman E. Tissue and Organ Transplantation. Ann Surg 1914;60:195-219. [Crossref] [PubMed]
- Guild WR, Harrison JH, Murray J, et al. Successful Homotransplantation of the Kidney in an Identical Twin. Trans Am Clin Climatol Assoc 1955-1956;67:167-73. [PubMed]
- Medawar PB. A second study of the behaviour and fate of skin homografts in rabbits: A Report to the War Wounds Committee of the Medical Research Council. J Anat 1945;79:157-76.4.
- Murray JE, Merrill JP, Dammin GJ, et al. Kidney Transplantation in Modified Recipients. Ann Surg 1962;156:337-55. [Crossref] [PubMed]
- Hamburger J, Vaysse J, Crosnier J, et al. Renal homotransplantation in man after radiation of the recipient. Experience with six patients since 1959. Am J Med 1962;32:854-71. [Crossref] [PubMed]
- Calne RY. The rejection of renal homografts. Inhibition in dogs by 6-mercaptopurine. Lancet 1960;1:417-8. [Crossref] [PubMed]
- Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979;2:1033-6. [Crossref] [PubMed]
- Starzl TE, Marchioro TL, Vonkaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659-76. [PubMed]
- Buchanan E. The operation: A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. Author: C N Barnard. S Afr Med J 2017;107:1041-4. [PubMed]
- Cooley DA, Frazier OH, Macris MP, et al. Heterotopic heart-single lung transplantation: report of a new technique. J Heart Transplant 1987;6:112-5. [PubMed]
- Toledo-Pereyra LH, Sutherland DER. Richard Carlton Lillehei Transplant and Shock Surgical Pioneer. J Invest Surg 2011;24:49-52. [Crossref] [PubMed]
- Goila AK, Pawar M. The diagnosis of brain death. Indian J Crit Care Med 2009;13:7. [Crossref] [PubMed]
- Cao Y, Shahrestani S, Chew H, et al. Donation after circulatory death for liver transplantation: A meta-analysis on the location of life support withdrawal affecting outcomes. Transplantation 2016;100:1513-24. [Crossref] [PubMed]
- Sass DA, Doyle AM. Liver and kidney transplantation. Med Clin North Am 2016;100:435-48. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network. National Data - OPTN. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed May 8, 2018.
- Jarrell BE, Kavic SM. NMS Surgery. Philadelphia: Wolters Kluwer, 2016.
- Merion RM, Sharma P, Mathur AK, et al. Evidence-based development of liver allocation: a review. Transpl Int 2011;24:965-72. [Crossref] [PubMed]
- Jochmans I, Akhtar MZ, Nasralla D, et al. Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant 2016;16:2545-55. [Crossref] [PubMed]
- Makowka L, Stieber AC, Sher L, et al. Surgical technique of orthotopic liver transplantation. Gastroenterol Clin North Am 1988;17:33-51. [PubMed]
- Miller C, Diago Uso T. The liver transplant operation. Clin Liver Dis 2013;2:192-6. [Crossref]
- Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation. Langenbecks Arch Chir 1988;373:127-30. [Crossref] [PubMed]
- Song AT, Avelino-Silva VI, Pecora RA, et al. Liver transplantation: fifty years of experience. World J Gastroenterol 2014;20:5363-74. [Crossref] [PubMed]
- Hong JC, Yersiz H, Busuttil RW. Where are we today in split liver transplantation? Curr Opin Organ Transplant 2011;16:269-73. [Crossref] [PubMed]
- Chan SC, Fan ST, Lo CM, et al. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg 2007;245:110-7. [Crossref] [PubMed]
- Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl 2011;17:1127-36. [Crossref] [PubMed]
- Seehofer D, Eurich D, Veltzke‐Schlieker W, et al. Biliary complications after liver transplantation: Old problems and new challenges. Am J Transplant 2013;13:253-65. [Crossref] [PubMed]
- Gad EH, Abdelsamee MA, Kamel Y. Hepatic arterial and portal venous complications after adult and pediatric living donor liver transplantation, risk factors, management and outcome (A retrospective cohort study). Ann Med Surg (Lond) 2016;8:28-39. [Crossref] [PubMed]
- Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: A systematic review and meta‐analysis. Liver Transpl 2014;20:425-36. [Crossref] [PubMed]
- Abradelo M, Sanabria R, Caso O, et al. Split liver transplantation: Where? when? how? Transplant Proc 2012;44:1513-6. [Crossref] [PubMed]
- Mogul DB, Luo X, Bowring MG, et al. Fifteen-year trends in pediatric liver transplants: Split, whole deceased, and living donor grafts. J Pediatr 2018;196:148-53.e2. [Crossref] [PubMed]
- Wang JH, Skeans MA, Israni AK. Current Status of Kidney Transplant Outcomes: Dying to Survive. Adv Chronic Kidney Dis 2016;23:281-6. [Crossref] [PubMed]
- Agerskov H, Bistrup C, Ludvigsen MS, et al. Experiences of living kidney donors during the donation process. J Ren Care 2018;44:96-105. [Crossref] [PubMed]
- Reese PP, Boudville N, Garg AX. Living kidney donation: Outcomes, ethics, and uncertainty. Lancet 2015;385:2003-13. [Crossref] [PubMed]
- Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the united states, 1988 to 1996. N Engl J Med 2000;342:605-12. [Crossref] [PubMed]
- Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data report: Kidney. Am J Transplant 2018;18:18-113. [Crossref] [PubMed]
- Rodrigue JR, Schold JD, Mandelbrot DA. The decline in living kidney donation in the United States: random variation or cause for concern? Transplantation 2013;96:767-73. [Crossref] [PubMed]
- Key Points: Living with Stage 4 Kidney Disease. The National Kidney Foundation. Available online: https://www.kidney.org/patients/peers/stage4. Published December 3, 2014. Accessed May 8, 2018.
- Transplant. The National Kidney Foundation. Available online: https://www.kidney.org/professionals/guidelines/guidelines_commentaries/transplant. Published September 13, 2016. Accessed May 8, 2018.
- Organ Procurement and Transplantation Network. National Data - OPTN. Available online: https://optn.transplant.hrsa.gov/resources/guidance/kidney-donor-profile-index-kdpi-guide-for-clinicians. Accessed May 8, 2018.
- Kasiske BL, Ramos EL, Gaston RS, et al. The evaluation of renal transplant candidates: clinical practice guidelines. Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol 1995;6:1-34. [PubMed]
- Segev DL, Gentry SE, Melancon JK, et al. Characterization of waiting times in a simulation of kidney paired donation. Am J Transplant 2005;5:2448-55. [Crossref] [PubMed]
- Thiruchelvam PT, Willicombe M, Hakim N, et al. Renal transplantation. BMJ 2011;343:d7300. [Crossref] [PubMed]
- Waller JR, Hiley AL, Mullin EJ, et al. Living kidney donation: a comparison of laparoscopic and conventional open operations. Postgrad Med J 2002;78:153-7. [Crossref] [PubMed]
- Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant 2011;11:2279-96. [Crossref] [PubMed]
- McLaren AJ, Jassem W, Gray D, et al. Delayed graft function: Risk factors and the relative effects of early function and acute rejection on long-term survival in cadaveric renal transplantation. Clin Transplant 1999;13:266-72. [Crossref] [PubMed]
- Dholakia S, Oskrochi Y, Easton G, et al. Advances in pancreas transplantation. J R Soc Med 2016;109:141-6. [Crossref] [PubMed]
- White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet 2009;373:1808-17. [Crossref] [PubMed]
- Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud 2011;8:6-16. [Crossref] [PubMed]
- Hameed AM, Wong G, Laurence JM, et al. A systematic review and meta-analysis of cold in situ perfusion and preservation for pancreas transplantation. HPB (Oxford) 2017;19:933-43. [Crossref] [PubMed]
- Kandaswamy R, Stock PG, Skeans MA, et al. OPTN/SRTR 2011 annual data report: Pancreas. Am J Transplant 2013;13:47-72. [Crossref] [PubMed]
- Han DJ, Sutherland DE. Pancreas transplantation. Gut Liver 2010;4:450-65. [Crossref] [PubMed]
- Corry RJ, Chakrabarti P, Shapiro R, et al. Comparison of enteric versus bladder drainage in pancreas transplantation. Transplant Proc 2001;33:1647-51. [Crossref] [PubMed]
- Senaratne NVS, Norris JM. Bladder vs enteric drainage following pancreatic transplantation: How best to support graft survival? A best evidence topic. Int J Surg 2015;22:149-52. [Crossref] [PubMed]
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001;233:463-501. [Crossref] [PubMed]
- Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830-5. [Crossref] [PubMed]
- Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant 2012;17:100-5. [Crossref] [PubMed]
- Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes 2010;59:1285-91. [Crossref] [PubMed]
- Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery 1972;72:175-86. [PubMed]
- Shapiro AM. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud 2012;9:385-406. [Crossref] [PubMed]
- Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230-8. [Crossref] [PubMed]
- Ontario Health Quality. Pancreas Islet Transplantation for Patients With Type 1 Diabetes Mellitus: A Clinical Evidence Review. Ont Health Technol Assess Ser 2015;15:1-84. [PubMed]
- Hatipoglu B. Islet Cell Transplantation and Alternative Therapies. Endocrinol Metab Clin North Am 2016;45:923-31. [Crossref] [PubMed]
- Chung J, Riella LV, Maillard I. Targeting the notch pathway to prevent rejection. Am J Transplant 2016;16:3079-85. [Crossref] [PubMed]
- Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol 2013;13:427-37. [Crossref] [PubMed]
- Wood S, Feng J, Chung J, et al. Transient blockade of delta-like Notch ligands prevents allograft rejection mediated by cellular and humoral mechanisms in a mouse model of heart transplantation. J Immunol 2015;194:2899-908. [Crossref] [PubMed]
- Mochizuki K, Xie F, He S, et al. Delta-like ligand 4 identifies a previously uncharacterized population of inflammatory dendritic cells that plays important roles in eliciting allogeneic T cell responses in mice. J Immunol 2013;190:3772-82. [Crossref] [PubMed]
- Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatol 2009;50:1247-57. [Crossref] [PubMed]
- Spitzer TR, Sykes M, Tolkoff-Rubin N, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation 2011;91:672-6. [Crossref] [PubMed]
- Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 2014;14:1599-611. [Crossref] [PubMed]
- Leventhal JR, Elliott M, Yolcu E, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation 2015;99:288-98. [Crossref] [PubMed]
- Scandling JD, Busque S, Shizuru JA, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant 2015;15:695-704. [Crossref] [PubMed]
- Bracho-Sanchez E, Xia CQ, Clare-Salzler MJ, et al. Micro and nano material carriers for immunomodulation. Am J Transplant 2016;16:3362-70. [Crossref] [PubMed]
- Lewis JS, Roche C, Zhang Y, et al. Combinatorial delivery of immunosuppressive factors to dendritic cells using dual-sized microspheres. J Mater Chem B 2014;2:2562-74. [Crossref] [PubMed]
- Cowan PJ, Tector AJ. The resurgence of xenotransplantation. Am J Transplant 2017;17:2531-6. [Crossref] [PubMed]
- Vadori M, Cozzi E. The immunological barriers to xenotransplantation. Tissue Antigens 2015;86:239-53. [Crossref] [PubMed]
- Petersen B, Frenzel A, Lucas-Hahn A, et al. Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes. Xenotransplantation 2016;23:338-46. [Crossref] [PubMed]
- Yang L, Guell M, Niu D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015;350:1101-4. [Crossref] [PubMed]
- Demange A, Yajjou-Hamalian H, Gallay K, et al. Porcine endogenous retrovirus-A/C: Biochemical properties of its integrase and susceptibility to raltegravir. J Gen Virol 2015;96:3124-30. [Crossref] [PubMed]
- Argaw T, Colon-Moran W, Wilson C. Susceptibility of porcine endogenous retrovirus to anti-retroviral inhibitors. Xenotransplantation 2016;23:151-8. [Crossref] [PubMed]


